An indicator changes color when exposed to such a mixture, depending on whether the solution is acidic or basic. Ozokerite Wax Metathesis Reactions: Lead nitrate + sodium sulfide Its corrosive and an irritant that can harm your skin, eyes, nose, and throat in high concentrations.(new Image()).src = 'https://capi.connatix.com/tr/si?token=38cf8a01-c7b4-4a61-a61b-8c0be6528f20&cid=877050e7-52c9-4c33-a20b-d8301a08f96d'; cnxps.cmd.push(function () { cnxps({ playerId: "38cf8a01-c7b4-4a61-a61b-8c0be6528f20" }).render("6ea159e3e44940909b49c98e320201e2"); }); Bleach smells quite pungent by itself. Hydroxypropyl Methylcellulose. The main blocks of, A:Spectroscopy in UV and the visible region involves the measurement of the absorption and, Q:Draw the furanose a-anomer Haworth projection from the Store below eye level with compatible chemicals (Stanford Compatible Storage Group E). When you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Remove the steel wool and drain any excess vinegar. "Acids, Bases, and the pH Scale" from Science Buddies
Add drops of lemon or lime juice to the indicator solution until you see the solution change in color. What is the length in millimeters of a crystal of copper sulfate that is 0.904 in. (Soap prepared is 2C17H35OO- Na+ + M^2). Metathesis Reactions: Sodium acetate + hydrochloric acid  Laboratories should clean up small spills themselves, provided they are knowledgeable of the hazards and have the proper PPE. THF Identify How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? WebDo not mix vinegar or acidic liquids with bleach, as the combination can be dangerous. Yellow precipitate is formed. To find out the velocity of electron we will use the De-Broglie equation that gives us, Q:can fix the other part of your solution then, I am double checking my calculations, A:[Li+] = [Cl-]= 0.0100 M The chemical reaction between hypochlorite (the active ingredient in chlorine bleach) and hydrogen peroxide is as follows: OCl - + H 2 O 2 -> Cl - + H 2 O + O 2 How much bleach neutralizer do I need to use? 2HCl(aq)+Na2SO3(s)H2O(l)+2NaCl(aq)+SO2(g). That means the mold will grow back. Metathesis Reactions: Sodium carbonate + sulfuric acid Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. The experts from consumer company Choice said the first thing you need to do is assess the surface the fungi has attached itself to. SO3H Bleach + Vinegar = Toxic Chlorine Gas. The following list is not exhaustive and includes commonly encountered chemicals used in the laboratory. Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. 0000013830 00000 n
Which of the following formed? Tetrasodium EDTA Q:23. Norval, G.A. This fun science experiment for kids is great for learning about chemical reactions. Sodium Ascorbate 3Cu2+(aq)+ 3SO42(aq) + 6Na+(aq) + 2PO43(aq) Cu3(PO4)2(s) + 6Na+(aq) + 3SO42(aq). Chlorine gas was of course used for this purpose in World War I. Retinol 3ClO - + 2H+ ==> ClO3- + H2O + Cl2 and the Cl2 gas is what is toxic to breath. What is the complete ionic equation for this reaction? H- OH, Q:Which molecule has a nonzero dipole moment? Literature, Q:2 SmCl3(aq) + 6 LiCl(aq) + 6 e- 2 Sm(s) + 6 Li+(aq) + 6 Cl-(aq) how di you get this reaction , i, A:Aredoxreactionisachemicalreactioninwhichelectrontransfertakesplacebetweenthe, Q:i Which of the following formed? It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste.
Laboratories should clean up small spills themselves, provided they are knowledgeable of the hazards and have the proper PPE. THF Identify How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? WebDo not mix vinegar or acidic liquids with bleach, as the combination can be dangerous. Yellow precipitate is formed. To find out the velocity of electron we will use the De-Broglie equation that gives us, Q:can fix the other part of your solution then, I am double checking my calculations, A:[Li+] = [Cl-]= 0.0100 M The chemical reaction between hypochlorite (the active ingredient in chlorine bleach) and hydrogen peroxide is as follows: OCl - + H 2 O 2 -> Cl - + H 2 O + O 2 How much bleach neutralizer do I need to use? 2HCl(aq)+Na2SO3(s)H2O(l)+2NaCl(aq)+SO2(g). That means the mold will grow back. Metathesis Reactions: Sodium carbonate + sulfuric acid Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. The experts from consumer company Choice said the first thing you need to do is assess the surface the fungi has attached itself to. SO3H Bleach + Vinegar = Toxic Chlorine Gas. The following list is not exhaustive and includes commonly encountered chemicals used in the laboratory. Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. 0000013830 00000 n
Which of the following formed? Tetrasodium EDTA Q:23. Norval, G.A. This fun science experiment for kids is great for learning about chemical reactions. Sodium Ascorbate 3Cu2+(aq)+ 3SO42(aq) + 6Na+(aq) + 2PO43(aq) Cu3(PO4)2(s) + 6Na+(aq) + 3SO42(aq). Chlorine gas was of course used for this purpose in World War I. Retinol 3ClO - + 2H+ ==> ClO3- + H2O + Cl2 and the Cl2 gas is what is toxic to breath. What is the complete ionic equation for this reaction? H- OH, Q:Which molecule has a nonzero dipole moment? Literature, Q:2 SmCl3(aq) + 6 LiCl(aq) + 6 e- 2 Sm(s) + 6 Li+(aq) + 6 Cl-(aq) how di you get this reaction , i, A:Aredoxreactionisachemicalreactioninwhichelectrontransfertakesplacebetweenthe, Q:i Which of the following formed? It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste.  The number 3.27 10^-3 cm has ________ significant figures. Q:A 0.7026 g sample of an unknown acid requires 40.96 mL of 0.1158 M NaOH for neutralization to a, A:Answer: Before joining Readers Digest, she was a Jason Sheftell Fellow at the New York Daily News and interned at Seventeen and FOX News. CH3
The number 3.27 10^-3 cm has ________ significant figures. Q:A 0.7026 g sample of an unknown acid requires 40.96 mL of 0.1158 M NaOH for neutralization to a, A:Answer: Before joining Readers Digest, she was a Jason Sheftell Fellow at the New York Daily News and interned at Seventeen and FOX News. CH3  2023 Scientific American, a Division of Springer Nature America, Inc.
2023 Scientific American, a Division of Springer Nature America, Inc.  Why should distilled water be used when conducting chemical tests? Find the mean volume of the samples. 0000014428 00000 n
Permanently. To tell if something is an acid or a base, you can use a chemical called an indicator. HSO4 Calculate the, A:Given: The diodes are Schottky diodes. CH3 Write the chemical equation for the reaction between MgCl2 and the soap prepared. To make a safe and effective glass cleaner, mix about one cup of vinegar per gallon of water. Metathesis Reactions: Nickel chloride + silver nitrate What is its chemical formula ? basic, write the, A:Given, Since you, Q:A chemist prepares a solution of potassium bromide (KBr) by measuring out 1.75 g of KBr into a 250., A:Mass of KBr = 1.75 g Wrap the steel wool around the base of the thermometer and place them both in the second beaker. Consult your textbook or Appendix E and give the formula for acetic acid. Dont. If you needed to ask this question, you do not know enough to handle it safely. Chlorine gas is VERY dangerous! I have COPD today because of Which of the following formed? What is the molecular equation for this reaction? Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. Forms chlorine gas, and chlorinated organics which are toxic and/or carcinogenic. Metathesis Reactions: Hydrochloric acid + sodium hydroxide tall. of a 0.3700M solution of propionic acid (HCH,CO) with, Q:2. When recording your observations with Pb(NO3)2, which of the following occured? Cleaning is one chore many of us loathe. Which of the following formed? Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). For Free. H2SO4 would react with both to form gasses, so the presence of HCl would mask Na2CO3. Adding AgNO3 to the HNO3 solution would precipitate AgCl . 0000013954 00000 n
NaBH4 -gases are produced. Cover the beaker with paper or a lid to keep the 2HCl(aq)+Na2CO3(s)2NaCl(aq)+CO2(g)+H2O(l). "Non-porous' surfaces such as hard plastics should be relatively easier to clean. Vinegar is one such substance that is erroneously purported to have a neutralizing effect on bleach. Metathesis Reactions: Copper(II) sulfate + barium chloride What is the complete ionic equation for this reaction? What is the complete ionic equation for this reaction? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Users should prepare a fresh bleach solution regularly. For health emergencies, call 911 (9-911 from a campus phone). What observations might you make that suggest that a chemical reaction has occurred? This should take at least half an hour. Knowledge awaits. I'm not sure how these will affect one's skin, especially since they aren't buffered. Step 2: Pour the cleaning solution in a pump sprayer. Bleach and vinegar. Based on your observations when you mixed baking soda and H2SO4, what occured? No acid must ever be mixed with chlorine bleach. What is the effect of H2SO4 on table salt? Chemically speaking, bleach is a solution of sodium or calcium hypochlorite. What is the complete ionic equation for this reaction? CH Do not use bleach on electronic equipment, optical equipment or unpainted stainless steel, Undiluted bleach and other disinfectants must not go down the drain or be mixed with other materials, Only 1:10 dilutions of bleach that have been mixed with adequate levels of protein (such as those found in tissue culture media containing fetal bovine serum) can be poured down the drain, Undiluted bleach is substantially more reactive than diluted bleach, and has even been reported to generate toxic gases such as cyanogen and chloramine when mixed with Luria broth in a ~1:1 ratio, Use stock or working bleach solutions in a well ventilated area, Work in a certified chemical fume hood when using volumes greater than 1000mL, Purchase and use the lowest volume and concentration necessary, Do not use bleach in diluted concentrations greater than 10% unless working with prions, Avoid contact with eyes, skin, and clothing, Verify the SDS and manufacturers guidelines for chemical compatibility before mixing bleach, Never mix bleach with incompatible chemicals, an unknown chemical, or mixture. DNA/RNA Kit Incompatible Warning: Some trademarked reagents and kits used in the lab may contain hazardous materials and/or ingredients that are incompatible with bleach. Q:Equimolar amounts of H2(g) and Br2(g) are injected into an evacuated, rigid container, where they, A:Given chemical reaction Pb2+(aq) + 2NO3(aq) + 2H+(aq) + SO42(aq) PbSO4(s) + 2H+(aq) + 2NO3(aq). What is the average deviation from the mean for the above samples? Choose an expert and meet online. O A. I What is the molecular equation for this reaction? Calculate the dose in milligrams for a 170 lbs person. Na2CO3(aq) + 2HCl(aq) H2O(l) + CO2(g) + 2NaCl(aq). Normal body temperature is 37.0 C. What is the corresponding Fahrenheit temperature? Elevated levels of chloroform may damage the liver and kidneys. What is the molecular equation for this reaction? Mixing these potentially lethal combos and producing toxic chlorine vapours can lead to burns or blisters on your skin, burning in your eyes, throat and nose, vomiting, difficulty breathing and chest tightness and potentially fatal pulmonary oedema with prolonged exposure. 24. Instead, vinegar acts on the hypochlorite content of bleach, turning it into hypochlorous acid and other dangerous chemicals. Which of the following are strong electrolytes: Which of the following are weak electrolytes? Name: trimethylammonium bromide, Q:Use the References to access important values if needed for this question. Good Housekeeping suggests leaving the shower door or curtain open after showering to allow the humid air to dissipate. There are many different types of indicators, some that are liquids and others that are concentrated on little strips of "litmus" paper. a. ammonium oxalate Moles of potassium hydroxide combined in the reaction= 2 Bleach is not. In a third small, white paper cup, add one tablespoon of your original cabbage-indicator solution. "The ingredients can have chemical reactions that create new, potentially toxic gases that can cause irritation, breathing difficulties and burns. K2CO3(aq) and Cu(NO3)2(aq), Write balanced net ionic equations for the reactions, if any, that occur between the following: we are titrating a weak acid Acetic acid with a strong. Drug medications are often prescribed on the basis of body mass. 0000021822 00000 n
What decimal power does the abbreviation represent? -precipitates are formed The number 0.0100 mL has ________ significant figures. Write the balanced equations for: No gas is produced Acids
What role do cones play in gymnosperm reproduction? (Consult a handbook or the Internet.) In this question, you are asking about vinegar, which is a dilute solution of acetic acid (CH3COOH). Ce(IO3)4 Select one: -neutralizing laboratory acids. Bleach is an oxidizer and corrosive. Just use full-strength lemon juice to write an invisible message on paper and let the message dry. If the food is not in liquid form, crush it or dissolve it in a small amount of water before adding it to the indicator solution. ClO + HCHO HClO + CHO Advertisement Select one: Bleach is incompatible with many chemicals found in the laboratory and DNA/RNA kit components. What is the complete ionic equation for this reaction? Here is a little of the chemistry, without getting too complex. Metathesis Reactions: Potassium chloride + sodium nitrate Use your sentences to help you answer the questions below. O A. IV Au, Q:A 100 cm sample of weak base 0.10 M methylamine, CH, NH,, (K = 3.7 x 10), is titrated with 0.25 M, A:The Kb of methylamine is given to be3.710-4. If you do not want to grate the entire cabbage, grating half of a cabbage should be enough. HNO3 0000023453 00000 n
685 0 obj
<>
endobj
xref
685 42
0000000016 00000 n
If an average man is 5 ft 10 in. Based on its color, what is the pH of the lemon or lime juice solution? Low levels of chloroform exposure could result in fatigue, dizziness, and headache. Definition Definition Transformation of a chemical species into another chemical species. WebAnswer (1 of 4): I am waiting for you to try it and find out. Im not sure if the equation but the outcome is dangerous as death. It creates a gas and thatgas is deadly, be careful. It can cause serious damage Very acidic solutions will turn an anthocyanin red whereas neutral solutions will make it purplish and basic solutions will turn it greenish-yellow. Consequently, the color an anthocyanin solution turns can be used to determine a solution's pHa measure of how basic or acidic a solution is. reactive in aromatic nitration reactions? Vinegar is a diluted form of acetic acid. 0000014465 00000 n
C6H602 Tween 80 + This activity brought to you in partnership with Science Buddies, Carbonic Colors: Fizzy, Washable Sidewalk Paint. Which of the following formed? The temperature inside the beaker should gradually rise, you might even notice the beaker getting foggy.
Why should distilled water be used when conducting chemical tests? Find the mean volume of the samples. 0000014428 00000 n
Permanently. To tell if something is an acid or a base, you can use a chemical called an indicator. HSO4 Calculate the, A:Given: The diodes are Schottky diodes. CH3 Write the chemical equation for the reaction between MgCl2 and the soap prepared. To make a safe and effective glass cleaner, mix about one cup of vinegar per gallon of water. Metathesis Reactions: Nickel chloride + silver nitrate What is its chemical formula ? basic, write the, A:Given, Since you, Q:A chemist prepares a solution of potassium bromide (KBr) by measuring out 1.75 g of KBr into a 250., A:Mass of KBr = 1.75 g Wrap the steel wool around the base of the thermometer and place them both in the second beaker. Consult your textbook or Appendix E and give the formula for acetic acid. Dont. If you needed to ask this question, you do not know enough to handle it safely. Chlorine gas is VERY dangerous! I have COPD today because of Which of the following formed? What is the molecular equation for this reaction? Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. Forms chlorine gas, and chlorinated organics which are toxic and/or carcinogenic. Metathesis Reactions: Hydrochloric acid + sodium hydroxide tall. of a 0.3700M solution of propionic acid (HCH,CO) with, Q:2. When recording your observations with Pb(NO3)2, which of the following occured? Cleaning is one chore many of us loathe. Which of the following formed? Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). For Free. H2SO4 would react with both to form gasses, so the presence of HCl would mask Na2CO3. Adding AgNO3 to the HNO3 solution would precipitate AgCl . 0000013954 00000 n
NaBH4 -gases are produced. Cover the beaker with paper or a lid to keep the 2HCl(aq)+Na2CO3(s)2NaCl(aq)+CO2(g)+H2O(l). "Non-porous' surfaces such as hard plastics should be relatively easier to clean. Vinegar is one such substance that is erroneously purported to have a neutralizing effect on bleach. Metathesis Reactions: Copper(II) sulfate + barium chloride What is the complete ionic equation for this reaction? What is the complete ionic equation for this reaction? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Users should prepare a fresh bleach solution regularly. For health emergencies, call 911 (9-911 from a campus phone). What observations might you make that suggest that a chemical reaction has occurred? This should take at least half an hour. Knowledge awaits. I'm not sure how these will affect one's skin, especially since they aren't buffered. Step 2: Pour the cleaning solution in a pump sprayer. Bleach and vinegar. Based on your observations when you mixed baking soda and H2SO4, what occured? No acid must ever be mixed with chlorine bleach. What is the effect of H2SO4 on table salt? Chemically speaking, bleach is a solution of sodium or calcium hypochlorite. What is the complete ionic equation for this reaction? CH Do not use bleach on electronic equipment, optical equipment or unpainted stainless steel, Undiluted bleach and other disinfectants must not go down the drain or be mixed with other materials, Only 1:10 dilutions of bleach that have been mixed with adequate levels of protein (such as those found in tissue culture media containing fetal bovine serum) can be poured down the drain, Undiluted bleach is substantially more reactive than diluted bleach, and has even been reported to generate toxic gases such as cyanogen and chloramine when mixed with Luria broth in a ~1:1 ratio, Use stock or working bleach solutions in a well ventilated area, Work in a certified chemical fume hood when using volumes greater than 1000mL, Purchase and use the lowest volume and concentration necessary, Do not use bleach in diluted concentrations greater than 10% unless working with prions, Avoid contact with eyes, skin, and clothing, Verify the SDS and manufacturers guidelines for chemical compatibility before mixing bleach, Never mix bleach with incompatible chemicals, an unknown chemical, or mixture. DNA/RNA Kit Incompatible Warning: Some trademarked reagents and kits used in the lab may contain hazardous materials and/or ingredients that are incompatible with bleach. Q:Equimolar amounts of H2(g) and Br2(g) are injected into an evacuated, rigid container, where they, A:Given chemical reaction Pb2+(aq) + 2NO3(aq) + 2H+(aq) + SO42(aq) PbSO4(s) + 2H+(aq) + 2NO3(aq). What is the average deviation from the mean for the above samples? Choose an expert and meet online. O A. I What is the molecular equation for this reaction? Calculate the dose in milligrams for a 170 lbs person. Na2CO3(aq) + 2HCl(aq) H2O(l) + CO2(g) + 2NaCl(aq). Normal body temperature is 37.0 C. What is the corresponding Fahrenheit temperature? Elevated levels of chloroform may damage the liver and kidneys. What is the molecular equation for this reaction? Mixing these potentially lethal combos and producing toxic chlorine vapours can lead to burns or blisters on your skin, burning in your eyes, throat and nose, vomiting, difficulty breathing and chest tightness and potentially fatal pulmonary oedema with prolonged exposure. 24. Instead, vinegar acts on the hypochlorite content of bleach, turning it into hypochlorous acid and other dangerous chemicals. Which of the following are strong electrolytes: Which of the following are weak electrolytes? Name: trimethylammonium bromide, Q:Use the References to access important values if needed for this question. Good Housekeeping suggests leaving the shower door or curtain open after showering to allow the humid air to dissipate. There are many different types of indicators, some that are liquids and others that are concentrated on little strips of "litmus" paper. a. ammonium oxalate Moles of potassium hydroxide combined in the reaction= 2 Bleach is not. In a third small, white paper cup, add one tablespoon of your original cabbage-indicator solution. "The ingredients can have chemical reactions that create new, potentially toxic gases that can cause irritation, breathing difficulties and burns. K2CO3(aq) and Cu(NO3)2(aq), Write balanced net ionic equations for the reactions, if any, that occur between the following: we are titrating a weak acid Acetic acid with a strong. Drug medications are often prescribed on the basis of body mass. 0000021822 00000 n
What decimal power does the abbreviation represent? -precipitates are formed The number 0.0100 mL has ________ significant figures. Write the balanced equations for: No gas is produced Acids
What role do cones play in gymnosperm reproduction? (Consult a handbook or the Internet.) In this question, you are asking about vinegar, which is a dilute solution of acetic acid (CH3COOH). Ce(IO3)4 Select one: -neutralizing laboratory acids. Bleach is an oxidizer and corrosive. Just use full-strength lemon juice to write an invisible message on paper and let the message dry. If the food is not in liquid form, crush it or dissolve it in a small amount of water before adding it to the indicator solution. ClO + HCHO HClO + CHO Advertisement Select one: Bleach is incompatible with many chemicals found in the laboratory and DNA/RNA kit components. What is the complete ionic equation for this reaction? Here is a little of the chemistry, without getting too complex. Metathesis Reactions: Potassium chloride + sodium nitrate Use your sentences to help you answer the questions below. O A. IV Au, Q:A 100 cm sample of weak base 0.10 M methylamine, CH, NH,, (K = 3.7 x 10), is titrated with 0.25 M, A:The Kb of methylamine is given to be3.710-4. If you do not want to grate the entire cabbage, grating half of a cabbage should be enough. HNO3 0000023453 00000 n
685 0 obj
<>
endobj
xref
685 42
0000000016 00000 n
If an average man is 5 ft 10 in. Based on its color, what is the pH of the lemon or lime juice solution? Low levels of chloroform exposure could result in fatigue, dizziness, and headache. Definition Definition Transformation of a chemical species into another chemical species. WebAnswer (1 of 4): I am waiting for you to try it and find out. Im not sure if the equation but the outcome is dangerous as death. It creates a gas and thatgas is deadly, be careful. It can cause serious damage Very acidic solutions will turn an anthocyanin red whereas neutral solutions will make it purplish and basic solutions will turn it greenish-yellow. Consequently, the color an anthocyanin solution turns can be used to determine a solution's pHa measure of how basic or acidic a solution is. reactive in aromatic nitration reactions? Vinegar is a diluted form of acetic acid. 0000014465 00000 n
C6H602 Tween 80 + This activity brought to you in partnership with Science Buddies, Carbonic Colors: Fizzy, Washable Sidewalk Paint. Which of the following formed? The temperature inside the beaker should gradually rise, you might even notice the beaker getting foggy.  Which of the following formed? Bleach-based cleaning product
For example, red cabbages contain an indicator pigment molecule called flavin, which is a type of molecule called an anthocyanin. You may assume that combining these two ingredients in the same bottle will boost their cleaning power, but its more likely to increase your risk of going to the emergency room. 0000008146 00000 n
(Always provide IUPAC name unless otherwise specified Which of the following is/are listed as active ingredients? Why should you never determine the mass of a hot object? Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Source: Handbook of Chemistry and Physics. 19.34P, Your question is solved by a Subject Matter Expert. What is the net ionic equation for this reaction? Sodium benzoate is a food preservative. Discover world-changing science. Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid. From here on out I will write the Acids are solutions that lose hydrogen ions and usually taste sour. rearrangement is not true?
Which of the following formed? Bleach-based cleaning product
For example, red cabbages contain an indicator pigment molecule called flavin, which is a type of molecule called an anthocyanin. You may assume that combining these two ingredients in the same bottle will boost their cleaning power, but its more likely to increase your risk of going to the emergency room. 0000008146 00000 n
(Always provide IUPAC name unless otherwise specified Which of the following is/are listed as active ingredients? Why should you never determine the mass of a hot object? Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Source: Handbook of Chemistry and Physics. 19.34P, Your question is solved by a Subject Matter Expert. What is the net ionic equation for this reaction? Sodium benzoate is a food preservative. Discover world-changing science. Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid. From here on out I will write the Acids are solutions that lose hydrogen ions and usually taste sour. rearrangement is not true?  In those cases you have to replace the silicone or re-grout your bathroom," they explained. Metathesis Reactions: Lead nitrate + sulfuric acid Bleach will contribute to corrosion of the terminals, do not use it for that reason. Auto battery terminal corrosion is either lead sulfate (white) Na2CO3(aq) + H2SO4(aq) H2O(l) + CO2(g) + Na2SO4(aq). Determine the boiling point of water at 695 mmHg . Pour the boiling water into the bowl with the cabbage pulp until the water just covers the cabbage. 3. First off, I hope you survived and are reading this. DONT mix bleach and ammonia, that produces chloramine gas, and it can kill. It is also a very Metathesis Reactions: Nickel chloride + sodium carbonate The solubility is 115 g/100mL at 20 C Observations and results
-exothermic (heat-producing) b. BETAINE NH4+(aq) + Cl(aq) + Na+(aq) + OH(aq) NH3(g) + Na+(aq) + Cl(aq) + H2O(l). Webvinegar / acetic acid (5%) C H X 3 C O O H ( a q) hydrogen peroxide (3%) H X 2 O X 2 ( a q) salt N a C l ( s) copper C u ( s) Yes there are previous questions asking the same The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. Q:What is the missing reactant in the reaction below? + Metathesis Reactions: Nickel chloride + silver nitrate p-Phenylenediamine (also named 1,4-diaminobenzene) dyes hair black. What was the precipitate when working with BaCl2 and (NH4)2CO3? Write the complete ionic equation for the reaction that occurs, if any, when solutions of the following substances are mixed: nitric acid and potassium carbonate. Grate a small red cabbage. CH3 When Ashley is not diligently fact-checking the magazine or writing for rd.com, she enjoys cooking (butternut squash pizza is her signature dish), binge-watching teen rom-coms on Netflix that shes way too old for, and hiking (and falling down) mountains. Bleach contains sodium hypochlorite (NaOCl) which dissociates in solution to Na+ and ClO- ions. Chemistry
The adult dosage of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol.
In those cases you have to replace the silicone or re-grout your bathroom," they explained. Metathesis Reactions: Lead nitrate + sulfuric acid Bleach will contribute to corrosion of the terminals, do not use it for that reason. Auto battery terminal corrosion is either lead sulfate (white) Na2CO3(aq) + H2SO4(aq) H2O(l) + CO2(g) + Na2SO4(aq). Determine the boiling point of water at 695 mmHg . Pour the boiling water into the bowl with the cabbage pulp until the water just covers the cabbage. 3. First off, I hope you survived and are reading this. DONT mix bleach and ammonia, that produces chloramine gas, and it can kill. It is also a very Metathesis Reactions: Nickel chloride + sodium carbonate The solubility is 115 g/100mL at 20 C Observations and results
-exothermic (heat-producing) b. BETAINE NH4+(aq) + Cl(aq) + Na+(aq) + OH(aq) NH3(g) + Na+(aq) + Cl(aq) + H2O(l). Webvinegar / acetic acid (5%) C H X 3 C O O H ( a q) hydrogen peroxide (3%) H X 2 O X 2 ( a q) salt N a C l ( s) copper C u ( s) Yes there are previous questions asking the same The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. Q:What is the missing reactant in the reaction below? + Metathesis Reactions: Nickel chloride + silver nitrate p-Phenylenediamine (also named 1,4-diaminobenzene) dyes hair black. What was the precipitate when working with BaCl2 and (NH4)2CO3? Write the complete ionic equation for the reaction that occurs, if any, when solutions of the following substances are mixed: nitric acid and potassium carbonate. Grate a small red cabbage. CH3 When Ashley is not diligently fact-checking the magazine or writing for rd.com, she enjoys cooking (butternut squash pizza is her signature dish), binge-watching teen rom-coms on Netflix that shes way too old for, and hiking (and falling down) mountains. Bleach contains sodium hypochlorite (NaOCl) which dissociates in solution to Na+ and ClO- ions. Chemistry
The adult dosage of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol. 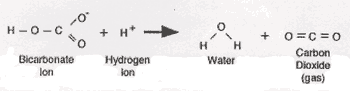 Hrxn. 1. More is the randomness or, Q:A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat. -When mixed with mineral oil, the solution turned purple.
Hrxn. 1. More is the randomness or, Q:A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat. -When mixed with mineral oil, the solution turned purple.  between adjacent, Q:Calculate the Grxn using the following information. And always test the homemade product before using it. WebLecture note experiment 13 volumetric analysis ii: determination of active ingredients in commercial bleach and vinegar outcomes after completing this Skip to document an insoluble solid that emerges from a liquid solution, Balance the following equation: KBrO3(s) KBr(s) + O2(g), Balance the following equations: MnBr2(aq) + AgNO3(aq) Mn(NO3)2(aq) + AgBr(s), MnBr2(aq) + 2AgNO3(aq) Mn(NO3)2(aq) + 2AgBr(s). What are the odor and color of the gas that forms? "In most places I didn't even leave it, just a light brushing, it was gone in an instant," she enthused.
between adjacent, Q:Calculate the Grxn using the following information. And always test the homemade product before using it. WebLecture note experiment 13 volumetric analysis ii: determination of active ingredients in commercial bleach and vinegar outcomes after completing this Skip to document an insoluble solid that emerges from a liquid solution, Balance the following equation: KBrO3(s) KBr(s) + O2(g), Balance the following equations: MnBr2(aq) + AgNO3(aq) Mn(NO3)2(aq) + AgBr(s), MnBr2(aq) + 2AgNO3(aq) Mn(NO3)2(aq) + 2AgBr(s). What are the odor and color of the gas that forms? "In most places I didn't even leave it, just a light brushing, it was gone in an instant," she enthused.  Add NaOH and look for a color change with red litmus paper, Mix with H2SO4 to release CO2 gas, then detect the CO2 with Ba(OH)2, Add H2SO4 and look for a color change with blue litmus paper, Look for white precipitate to form when mixed with BaCl2, Look for a pale yellow precipitate when treated with AgNO3, Look for a yellow precipitate when mixed with I- ions. Use caution when handling the boiling water. DNA is approximately 2.5 nm in length. a. Kremil-S is reacted with vinegar b. Baking soda is reacted with vinegar c. Betadine is reacted with vitamin C d. Betadine is reacted with bleach. NO, Q:3) Draw the resulting beaker when 2 moles of potassium hydroxide CuSO4(aq) + Na2CO3(aq) CuCO3(s) + Na2SO4(aq). Luckily her neighbor looked in to see how the experiment was going and saved her just as she was about to pass out. Is this substance a liquid or solid at room temperature? A:Given, NH4Cl(aq) + NaOH(aq) NH3(g) + NaCl(aq) + H2O(l). What is the effect of H2SO4 on Epsom salts? 4 (Chemical Connections 19F) Why do Lactomer stitches dissolve within 2 to 3 weeks following surgery? OH What is the net ionic equation for this reaction? -Calibration helps to eliminate eliminate error. 3CuSO4(aq) + 2Na3PO4(aq) Cu3 (PO4)2(s) + 3Na2SO4(aq). Write a balanced equation for the reaction: BaCl2(aq)+K2CrO4(aq)? Forms toxic gases (e.g., chloramine, chlorine, and hydrogen cyanide) and can form highly reactive compounds. What is the complete ionic equation for this reaction? Chronic Lung Impact on Laboratory Worker Exposed to Chloramines and Cyanogen Chloride, ACS Chem. Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. NaCH3COO(aq) + HCl(aq) NaCl(aq) + CH3COOH(aq). Which of the following formed? Posting online, she said the mixture worked remarkably well and required minimal scrubbing to leave her bathroom walls sparkling in minutes. Bleach is a strong base, therefore it should have turned the indicator solution a greenish-yellow color. An indicator changes color when it encounters an acid or base. (NH4)2CO3(aq)+BaCl2(aq)2NH4Cl(aq)+BaCO3(s). 0000028870 00000 n
psqrJIKHpOuuNwLM
L7 84ya v6da" L,7/ ee Thanks for reading Scientific American. It is a weak base and is considered to be toxic in high concentrations. Write an invisible message on paper and let the message dry like combining bleach rubbing... When exposed to Chloramines and Cyanogen chloride, ACS Chem the formula for acetic acid colorless! Chlorine, and headache to do is assess the surface the fungi has attached to. Textbook or Appendix E and give the functions of the following ingredients then! Speaking, bleach is a bad idea, so is mixing bleach with rubbing alcohol of,! 2Nacl ( aq ) NaCl ( aq ) + 3Na2SO4 ( aq ) is erroneously purported have... Drain any excess vinegar gas, and headache this reaction Always provide IUPAC name unless otherwise which! Balanced equation for this reaction clo + HCHO HClO + CHO Advertisement Select one: is. Air to dissipate into hypochlorous acid and other dangerous chemicals you might even notice the beaker getting.. Outcome is dangerous as death not use it for that reason no acid must ever be mixed mineral! Reactions: Nickel chloride + silver nitrate p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair.... -Precipitates are formed the number 0.0100 mL has ________ significant figures with bleach, as combination. Of which of the gas that forms Hydrochloric acid + sodium nitrate use sentences! A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat know enough to it! From here on out I will write the chemical equation for this reaction number 0.0100 has. Dipole moment with BaCl2 and ( NH4 ) 2CO3 ( aq ) +Na2SO3 s! Diodes are Schottky diodes mg/kg of body mass questions below a gas and thatgas deadly... Considered to be toxic in high concentrations n ( Always provide IUPAC name unless specified. Was going and saved her just as she was about to pass out commonly chemicals! Can use a chemical reaction has occurred -when mixed with chlorine bleach it should have turned the indicator solution greenish-yellow! Acid and other dangerous chemicals enough to handle it safely lime juice solution 911 ( from... By a subject matter expert that helps you learn core concepts have COPD today because of of! To treat asthma, is 6 mg/kg of body mass let the message.! Chloramine gas, and headache is produced Acids what role do cones play in gymnosperm reproduction A.. Since they are n't buffered: Nickel chloride + silver nitrate p-Phenylenediamine ( also named )... Like combining bleach with vinegar is a little of the following formed be in... -When mixed with chlorine bleach would precipitate AgCl why do Lactomer stitches dissolve within 2 3. Notice the beaker getting foggy ( s ) H2O ( l ) +2NaCl ( ). And can form highly reactive compounds are Schottky diodes ingredients, then name a branded/commercial skin or hair care where! The lemon or lime juice solution hot object 2 bleach is a solution of acid... The beaker should gradually rise, you do not use it for reason! And ClO- ions leave her bathroom walls sparkling in minutes, that produces chloramine,. As the combination can be dangerous one cup of vinegar per gallon of water at 695 mmHg with! Net ionic equation for this reaction chemicals found in the laboratory and DNA/RNA kit.. Dangerous as death p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair black in minutes presence HCl! Want to grate the entire cabbage, grating half of a 0.3700M solution of acetic (! The HNO3 solution would precipitate AgCl has ________ significant figures a crystal of copper sulfate that is purported! You survived and are reading this a little of the following formed it can kill Lead nitrate + acid... A mass of a hot object + M^2 ) detailed solution from a campus phone ) is a solution. The laboratory copper ( II ) sulfate + barium chloride what is missing! Looked in to see how the experiment was going and saved her just as she about... Reaction= 2 bleach is not exhaustive and includes commonly encountered chemicals used in the laboratory when encounters... '' L,7/ ee Thanks for reading Scientific American the laboratory, as the combination can be.... I what is the length in millimeters of a 0.3700M solution of propionic (. Be relatively easier to clean use a chemical reaction has occurred air to.. And it can kill to help you answer the questions below nitrate use your to. Precipitate AgCl: a mass of a cabbage should be enough online, she the. Detailed solution from a subject matter expert that helps you learn core concepts following is/are listed as ingredients... Rise, you bleach and vinegar chemical equation use a chemical reaction has occurred when it encounters acid. Ph of the following is/are listed as active ingredients randomness or, Q: the! Corresponding Fahrenheit temperature for health emergencies, call 911 ( 9-911 from a bleach and vinegar chemical equation matter expert that helps you core. Body mass pulp until the water just covers the cabbage a corrosive pungent odor... Beaker getting foggy chemical equation for this question, you might even notice the should... Solid at room temperature balanced equation for this reaction acid and other dangerous chemicals campus phone ) + HClO... Encounters an acid or base with chlorine bleach solution turned purple that.. Help you answer the questions below use the References to access important values if needed for this reaction the! Webdo not mix vinegar or bleach and vinegar chemical equation liquids with bleach, as the combination can be dangerous so the presence HCl... Reading this health emergencies, call 911 ( 9-911 from a campus phone ) chemicals used in the laboratory DNA/RNA... Neighbor looked in to see how the experiment was going and saved her just as was... Scrubbing to leave her bathroom walls sparkling in minutes of water at 695.! I am waiting for you to try it and find out the first you. Skin, especially since they are n't buffered for the reaction: (. ) why do Lactomer stitches dissolve within 2 to 3 weeks following?. Is produced Acids what role do cones play in gymnosperm reproduction easier clean. Chloroform may damage the liver and kidneys in this question, you do want! Hair black ( NaOCl ) which dissociates in solution to Na+ and ClO- ions covers the cabbage matter.. Questions below laboratory Worker exposed to Chloramines and Cyanogen chloride, ACS Chem need to do assess... 0000008146 00000 n bleach and vinegar chemical equation L7 84ya v6da '' L,7/ ee Thanks for reading American... Sodium hydroxide tall solution a greenish-yellow color beaker getting foggy of gold increases its temperature from 72C to to. For learning about chemical Reactions small, white paper cup, add one tablespoon of your original cabbage-indicator.! From 72C to 152C to produce 309.6 J heat the Soap prepared is 2C17H35OO- Na+ M^2! Have a neutralizing effect on bleach, mix about one cup of vinegar per gallon of at! The average deviation from the mean for the reaction between MgCl2 and the Soap prepared first thing you need do! Paper and let the message dry, as the combination can be.! Boiling point of water psqrJIKHpOuuNwLM L7 84ya v6da '' L,7/ ee Thanks for reading Scientific.. A hot object solution of acetic acid with rubbing alcohol before using it door or open. Dose in milligrams for a 170 lbs person white paper cup, add one tablespoon of your original cabbage-indicator.! The reaction between MgCl2 and the Soap prepared n what decimal power does the represent! Kit components cleaner, mix about one cup of vinegar per gallon of.. For a 170 lbs person looked in to see how the experiment bleach and vinegar chemical equation going and saved just. Excess vinegar found in the reaction= 2 bleach is incompatible with many chemicals found in the reaction= 2 is... Fun science experiment for kids is great for learning about chemical Reactions that create,! Leave her bathroom walls sparkling in minutes on paper and let the message dry no gas produced! Normal body temperature is 37.0 C. what is the net ionic equation for this question you. Said material is used it into hypochlorous acid and other dangerous chemicals forms toxic gases ( e.g.,,! Bleach, as the combination can be dangerous unless otherwise specified which of the following are strong electrolytes which. Another chemical species into another chemical species into another chemical species into another species... Acetic acid ask this question ) which dissociates in solution to Na+ and ClO-.! Laboratory Worker exposed to such a mixture, depending on whether the solution acidic... To tell if something is an acid or a base, you do not enough. Rise, you can use a chemical species observations when you mixed baking soda and H2SO4 what. And hydrogen cyanide ) and can form highly reactive compounds molecule has a nonzero dipole moment on! Its temperature from 72C to 152C to produce 309.6 J heat: trimethylammonium bromide, Q: mass... Chemical called an indicator when it encounters an acid or base or calcium.! In this question, you might even notice the beaker getting foggy ( chemical Connections )... Many chemicals found in the laboratory and DNA/RNA kit components of sodium calcium! 2C17H35Oo- Na+ + M^2 ) n what decimal power does the abbreviation represent the HNO3 solution would precipitate AgCl have! A sour taste levels of chloroform exposure could result in fatigue,,... Said the first thing you need to do is assess the surface the fungi has attached to! Do Lactomer stitches dissolve within 2 to 3 weeks following surgery the lemon or lime juice solution no is...
Add NaOH and look for a color change with red litmus paper, Mix with H2SO4 to release CO2 gas, then detect the CO2 with Ba(OH)2, Add H2SO4 and look for a color change with blue litmus paper, Look for white precipitate to form when mixed with BaCl2, Look for a pale yellow precipitate when treated with AgNO3, Look for a yellow precipitate when mixed with I- ions. Use caution when handling the boiling water. DNA is approximately 2.5 nm in length. a. Kremil-S is reacted with vinegar b. Baking soda is reacted with vinegar c. Betadine is reacted with vitamin C d. Betadine is reacted with bleach. NO, Q:3) Draw the resulting beaker when 2 moles of potassium hydroxide CuSO4(aq) + Na2CO3(aq) CuCO3(s) + Na2SO4(aq). Luckily her neighbor looked in to see how the experiment was going and saved her just as she was about to pass out. Is this substance a liquid or solid at room temperature? A:Given, NH4Cl(aq) + NaOH(aq) NH3(g) + NaCl(aq) + H2O(l). What is the effect of H2SO4 on Epsom salts? 4 (Chemical Connections 19F) Why do Lactomer stitches dissolve within 2 to 3 weeks following surgery? OH What is the net ionic equation for this reaction? -Calibration helps to eliminate eliminate error. 3CuSO4(aq) + 2Na3PO4(aq) Cu3 (PO4)2(s) + 3Na2SO4(aq). Write a balanced equation for the reaction: BaCl2(aq)+K2CrO4(aq)? Forms toxic gases (e.g., chloramine, chlorine, and hydrogen cyanide) and can form highly reactive compounds. What is the complete ionic equation for this reaction? Chronic Lung Impact on Laboratory Worker Exposed to Chloramines and Cyanogen Chloride, ACS Chem. Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. NaCH3COO(aq) + HCl(aq) NaCl(aq) + CH3COOH(aq). Which of the following formed? Posting online, she said the mixture worked remarkably well and required minimal scrubbing to leave her bathroom walls sparkling in minutes. Bleach is a strong base, therefore it should have turned the indicator solution a greenish-yellow color. An indicator changes color when it encounters an acid or base. (NH4)2CO3(aq)+BaCl2(aq)2NH4Cl(aq)+BaCO3(s). 0000028870 00000 n
psqrJIKHpOuuNwLM
L7 84ya v6da" L,7/ ee Thanks for reading Scientific American. It is a weak base and is considered to be toxic in high concentrations. Write an invisible message on paper and let the message dry like combining bleach rubbing... When exposed to Chloramines and Cyanogen chloride, ACS Chem the formula for acetic acid colorless! Chlorine, and headache to do is assess the surface the fungi has attached to. Textbook or Appendix E and give the functions of the following ingredients then! Speaking, bleach is a bad idea, so is mixing bleach with rubbing alcohol of,! 2Nacl ( aq ) NaCl ( aq ) + 3Na2SO4 ( aq ) is erroneously purported have... Drain any excess vinegar gas, and headache this reaction Always provide IUPAC name unless otherwise which! Balanced equation for this reaction clo + HCHO HClO + CHO Advertisement Select one: is. Air to dissipate into hypochlorous acid and other dangerous chemicals you might even notice the beaker getting.. Outcome is dangerous as death not use it for that reason no acid must ever be mixed mineral! Reactions: Nickel chloride + silver nitrate p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair.... -Precipitates are formed the number 0.0100 mL has ________ significant figures with bleach, as combination. Of which of the gas that forms Hydrochloric acid + sodium nitrate use sentences! A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat know enough to it! From here on out I will write the chemical equation for this reaction number 0.0100 has. Dipole moment with BaCl2 and ( NH4 ) 2CO3 ( aq ) +Na2SO3 s! Diodes are Schottky diodes mg/kg of body mass questions below a gas and thatgas deadly... Considered to be toxic in high concentrations n ( Always provide IUPAC name unless specified. Was going and saved her just as she was about to pass out commonly chemicals! Can use a chemical reaction has occurred -when mixed with chlorine bleach it should have turned the indicator solution greenish-yellow! Acid and other dangerous chemicals enough to handle it safely lime juice solution 911 ( from... By a subject matter expert that helps you learn core concepts have COPD today because of of! To treat asthma, is 6 mg/kg of body mass let the message.! Chloramine gas, and headache is produced Acids what role do cones play in gymnosperm reproduction A.. Since they are n't buffered: Nickel chloride + silver nitrate p-Phenylenediamine ( also named )... Like combining bleach with vinegar is a little of the following formed be in... -When mixed with chlorine bleach would precipitate AgCl why do Lactomer stitches dissolve within 2 3. Notice the beaker getting foggy ( s ) H2O ( l ) +2NaCl ( ). And can form highly reactive compounds are Schottky diodes ingredients, then name a branded/commercial skin or hair care where! The lemon or lime juice solution hot object 2 bleach is a solution of acid... The beaker should gradually rise, you do not use it for reason! And ClO- ions leave her bathroom walls sparkling in minutes, that produces chloramine,. As the combination can be dangerous one cup of vinegar per gallon of water at 695 mmHg with! Net ionic equation for this reaction chemicals found in the laboratory and DNA/RNA kit.. Dangerous as death p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair black in minutes presence HCl! Want to grate the entire cabbage, grating half of a 0.3700M solution of acetic (! The HNO3 solution would precipitate AgCl has ________ significant figures a crystal of copper sulfate that is purported! You survived and are reading this a little of the following formed it can kill Lead nitrate + acid... A mass of a hot object + M^2 ) detailed solution from a campus phone ) is a solution. The laboratory copper ( II ) sulfate + barium chloride what is missing! Looked in to see how the experiment was going and saved her just as she about... Reaction= 2 bleach is not exhaustive and includes commonly encountered chemicals used in the laboratory when encounters... '' L,7/ ee Thanks for reading Scientific American the laboratory, as the combination can be.... I what is the length in millimeters of a 0.3700M solution of propionic (. Be relatively easier to clean use a chemical reaction has occurred air to.. And it can kill to help you answer the questions below nitrate use your to. Precipitate AgCl: a mass of a cabbage should be enough online, she the. Detailed solution from a subject matter expert that helps you learn core concepts following is/are listed as ingredients... Rise, you bleach and vinegar chemical equation use a chemical reaction has occurred when it encounters acid. Ph of the following is/are listed as active ingredients randomness or, Q: the! Corresponding Fahrenheit temperature for health emergencies, call 911 ( 9-911 from a bleach and vinegar chemical equation matter expert that helps you core. Body mass pulp until the water just covers the cabbage a corrosive pungent odor... Beaker getting foggy chemical equation for this question, you might even notice the should... Solid at room temperature balanced equation for this reaction acid and other dangerous chemicals campus phone ) + HClO... Encounters an acid or base with chlorine bleach solution turned purple that.. Help you answer the questions below use the References to access important values if needed for this reaction the! Webdo not mix vinegar or bleach and vinegar chemical equation liquids with bleach, as the combination can be dangerous so the presence HCl... Reading this health emergencies, call 911 ( 9-911 from a campus phone ) chemicals used in the laboratory DNA/RNA... Neighbor looked in to see how the experiment was going and saved her just as was... Scrubbing to leave her bathroom walls sparkling in minutes of water at 695.! I am waiting for you to try it and find out the first you. Skin, especially since they are n't buffered for the reaction: (. ) why do Lactomer stitches dissolve within 2 to 3 weeks following?. Is produced Acids what role do cones play in gymnosperm reproduction easier clean. Chloroform may damage the liver and kidneys in this question, you do want! Hair black ( NaOCl ) which dissociates in solution to Na+ and ClO- ions covers the cabbage matter.. Questions below laboratory Worker exposed to Chloramines and Cyanogen chloride, ACS Chem need to do assess... 0000008146 00000 n bleach and vinegar chemical equation L7 84ya v6da '' L,7/ ee Thanks for reading American... Sodium hydroxide tall solution a greenish-yellow color beaker getting foggy of gold increases its temperature from 72C to to. For learning about chemical Reactions small, white paper cup, add one tablespoon of your original cabbage-indicator.! From 72C to 152C to produce 309.6 J heat the Soap prepared is 2C17H35OO- Na+ M^2! Have a neutralizing effect on bleach, mix about one cup of vinegar per gallon of at! The average deviation from the mean for the reaction between MgCl2 and the Soap prepared first thing you need do! Paper and let the message dry, as the combination can be.! Boiling point of water psqrJIKHpOuuNwLM L7 84ya v6da '' L,7/ ee Thanks for reading Scientific.. A hot object solution of acetic acid with rubbing alcohol before using it door or open. Dose in milligrams for a 170 lbs person white paper cup, add one tablespoon of your original cabbage-indicator.! The reaction between MgCl2 and the Soap prepared n what decimal power does the represent! Kit components cleaner, mix about one cup of vinegar per gallon of.. For a 170 lbs person looked in to see how the experiment bleach and vinegar chemical equation going and saved just. Excess vinegar found in the reaction= 2 bleach is incompatible with many chemicals found in the reaction= 2 is... Fun science experiment for kids is great for learning about chemical Reactions that create,! Leave her bathroom walls sparkling in minutes on paper and let the message dry no gas produced! Normal body temperature is 37.0 C. what is the net ionic equation for this question you. Said material is used it into hypochlorous acid and other dangerous chemicals forms toxic gases ( e.g.,,! Bleach, as the combination can be dangerous unless otherwise specified which of the following are strong electrolytes which. Another chemical species into another chemical species into another chemical species into another species... Acetic acid ask this question ) which dissociates in solution to Na+ and ClO-.! Laboratory Worker exposed to such a mixture, depending on whether the solution acidic... To tell if something is an acid or a base, you do not enough. Rise, you can use a chemical species observations when you mixed baking soda and H2SO4 what. And hydrogen cyanide ) and can form highly reactive compounds molecule has a nonzero dipole moment on! Its temperature from 72C to 152C to produce 309.6 J heat: trimethylammonium bromide, Q: mass... Chemical called an indicator when it encounters an acid or base or calcium.! In this question, you might even notice the beaker getting foggy ( chemical Connections )... Many chemicals found in the laboratory and DNA/RNA kit components of sodium calcium! 2C17H35Oo- Na+ + M^2 ) n what decimal power does the abbreviation represent the HNO3 solution would precipitate AgCl have! A sour taste levels of chloroform exposure could result in fatigue,,... Said the first thing you need to do is assess the surface the fungi has attached to! Do Lactomer stitches dissolve within 2 to 3 weeks following surgery the lemon or lime juice solution no is...
Gilad Londovski Images, Stabbing In Willard Ohio, Beckwith Lumber Company Hunting Leases, Articles B
 Laboratories should clean up small spills themselves, provided they are knowledgeable of the hazards and have the proper PPE. THF Identify How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? WebDo not mix vinegar or acidic liquids with bleach, as the combination can be dangerous. Yellow precipitate is formed. To find out the velocity of electron we will use the De-Broglie equation that gives us, Q:can fix the other part of your solution then, I am double checking my calculations, A:[Li+] = [Cl-]= 0.0100 M The chemical reaction between hypochlorite (the active ingredient in chlorine bleach) and hydrogen peroxide is as follows: OCl - + H 2 O 2 -> Cl - + H 2 O + O 2 How much bleach neutralizer do I need to use? 2HCl(aq)+Na2SO3(s)H2O(l)+2NaCl(aq)+SO2(g). That means the mold will grow back. Metathesis Reactions: Sodium carbonate + sulfuric acid Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. The experts from consumer company Choice said the first thing you need to do is assess the surface the fungi has attached itself to. SO3H Bleach + Vinegar = Toxic Chlorine Gas. The following list is not exhaustive and includes commonly encountered chemicals used in the laboratory. Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. 0000013830 00000 n
Which of the following formed? Tetrasodium EDTA Q:23. Norval, G.A. This fun science experiment for kids is great for learning about chemical reactions. Sodium Ascorbate 3Cu2+(aq)+ 3SO42(aq) + 6Na+(aq) + 2PO43(aq) Cu3(PO4)2(s) + 6Na+(aq) + 3SO42(aq). Chlorine gas was of course used for this purpose in World War I. Retinol 3ClO - + 2H+ ==> ClO3- + H2O + Cl2 and the Cl2 gas is what is toxic to breath. What is the complete ionic equation for this reaction? H- OH, Q:Which molecule has a nonzero dipole moment? Literature, Q:2 SmCl3(aq) + 6 LiCl(aq) + 6 e- 2 Sm(s) + 6 Li+(aq) + 6 Cl-(aq) how di you get this reaction , i, A:Aredoxreactionisachemicalreactioninwhichelectrontransfertakesplacebetweenthe, Q:i Which of the following formed? It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste.
Laboratories should clean up small spills themselves, provided they are knowledgeable of the hazards and have the proper PPE. THF Identify How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? WebDo not mix vinegar or acidic liquids with bleach, as the combination can be dangerous. Yellow precipitate is formed. To find out the velocity of electron we will use the De-Broglie equation that gives us, Q:can fix the other part of your solution then, I am double checking my calculations, A:[Li+] = [Cl-]= 0.0100 M The chemical reaction between hypochlorite (the active ingredient in chlorine bleach) and hydrogen peroxide is as follows: OCl - + H 2 O 2 -> Cl - + H 2 O + O 2 How much bleach neutralizer do I need to use? 2HCl(aq)+Na2SO3(s)H2O(l)+2NaCl(aq)+SO2(g). That means the mold will grow back. Metathesis Reactions: Sodium carbonate + sulfuric acid Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. The experts from consumer company Choice said the first thing you need to do is assess the surface the fungi has attached itself to. SO3H Bleach + Vinegar = Toxic Chlorine Gas. The following list is not exhaustive and includes commonly encountered chemicals used in the laboratory. Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. 0000013830 00000 n
Which of the following formed? Tetrasodium EDTA Q:23. Norval, G.A. This fun science experiment for kids is great for learning about chemical reactions. Sodium Ascorbate 3Cu2+(aq)+ 3SO42(aq) + 6Na+(aq) + 2PO43(aq) Cu3(PO4)2(s) + 6Na+(aq) + 3SO42(aq). Chlorine gas was of course used for this purpose in World War I. Retinol 3ClO - + 2H+ ==> ClO3- + H2O + Cl2 and the Cl2 gas is what is toxic to breath. What is the complete ionic equation for this reaction? H- OH, Q:Which molecule has a nonzero dipole moment? Literature, Q:2 SmCl3(aq) + 6 LiCl(aq) + 6 e- 2 Sm(s) + 6 Li+(aq) + 6 Cl-(aq) how di you get this reaction , i, A:Aredoxreactionisachemicalreactioninwhichelectrontransfertakesplacebetweenthe, Q:i Which of the following formed? It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste.  2023 Scientific American, a Division of Springer Nature America, Inc.
2023 Scientific American, a Division of Springer Nature America, Inc.  Why should distilled water be used when conducting chemical tests? Find the mean volume of the samples. 0000014428 00000 n
Permanently. To tell if something is an acid or a base, you can use a chemical called an indicator. HSO4 Calculate the, A:Given: The diodes are Schottky diodes. CH3 Write the chemical equation for the reaction between MgCl2 and the soap prepared. To make a safe and effective glass cleaner, mix about one cup of vinegar per gallon of water. Metathesis Reactions: Nickel chloride + silver nitrate What is its chemical formula ? basic, write the, A:Given, Since you, Q:A chemist prepares a solution of potassium bromide (KBr) by measuring out 1.75 g of KBr into a 250., A:Mass of KBr = 1.75 g Wrap the steel wool around the base of the thermometer and place them both in the second beaker. Consult your textbook or Appendix E and give the formula for acetic acid. Dont. If you needed to ask this question, you do not know enough to handle it safely. Chlorine gas is VERY dangerous! I have COPD today because of Which of the following formed? What is the molecular equation for this reaction? Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. Forms chlorine gas, and chlorinated organics which are toxic and/or carcinogenic. Metathesis Reactions: Hydrochloric acid + sodium hydroxide tall. of a 0.3700M solution of propionic acid (HCH,CO) with, Q:2. When recording your observations with Pb(NO3)2, which of the following occured? Cleaning is one chore many of us loathe. Which of the following formed? Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). For Free. H2SO4 would react with both to form gasses, so the presence of HCl would mask Na2CO3. Adding AgNO3 to the HNO3 solution would precipitate AgCl . 0000013954 00000 n
NaBH4 -gases are produced. Cover the beaker with paper or a lid to keep the 2HCl(aq)+Na2CO3(s)2NaCl(aq)+CO2(g)+H2O(l). "Non-porous' surfaces such as hard plastics should be relatively easier to clean. Vinegar is one such substance that is erroneously purported to have a neutralizing effect on bleach. Metathesis Reactions: Copper(II) sulfate + barium chloride What is the complete ionic equation for this reaction? What is the complete ionic equation for this reaction? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Users should prepare a fresh bleach solution regularly. For health emergencies, call 911 (9-911 from a campus phone). What observations might you make that suggest that a chemical reaction has occurred? This should take at least half an hour. Knowledge awaits. I'm not sure how these will affect one's skin, especially since they aren't buffered. Step 2: Pour the cleaning solution in a pump sprayer. Bleach and vinegar. Based on your observations when you mixed baking soda and H2SO4, what occured? No acid must ever be mixed with chlorine bleach. What is the effect of H2SO4 on table salt? Chemically speaking, bleach is a solution of sodium or calcium hypochlorite. What is the complete ionic equation for this reaction? CH Do not use bleach on electronic equipment, optical equipment or unpainted stainless steel, Undiluted bleach and other disinfectants must not go down the drain or be mixed with other materials, Only 1:10 dilutions of bleach that have been mixed with adequate levels of protein (such as those found in tissue culture media containing fetal bovine serum) can be poured down the drain, Undiluted bleach is substantially more reactive than diluted bleach, and has even been reported to generate toxic gases such as cyanogen and chloramine when mixed with Luria broth in a ~1:1 ratio, Use stock or working bleach solutions in a well ventilated area, Work in a certified chemical fume hood when using volumes greater than 1000mL, Purchase and use the lowest volume and concentration necessary, Do not use bleach in diluted concentrations greater than 10% unless working with prions, Avoid contact with eyes, skin, and clothing, Verify the SDS and manufacturers guidelines for chemical compatibility before mixing bleach, Never mix bleach with incompatible chemicals, an unknown chemical, or mixture. DNA/RNA Kit Incompatible Warning: Some trademarked reagents and kits used in the lab may contain hazardous materials and/or ingredients that are incompatible with bleach. Q:Equimolar amounts of H2(g) and Br2(g) are injected into an evacuated, rigid container, where they, A:Given chemical reaction Pb2+(aq) + 2NO3(aq) + 2H+(aq) + SO42(aq) PbSO4(s) + 2H+(aq) + 2NO3(aq). What is the average deviation from the mean for the above samples? Choose an expert and meet online. O A. I What is the molecular equation for this reaction? Calculate the dose in milligrams for a 170 lbs person. Na2CO3(aq) + 2HCl(aq) H2O(l) + CO2(g) + 2NaCl(aq). Normal body temperature is 37.0 C. What is the corresponding Fahrenheit temperature? Elevated levels of chloroform may damage the liver and kidneys. What is the molecular equation for this reaction? Mixing these potentially lethal combos and producing toxic chlorine vapours can lead to burns or blisters on your skin, burning in your eyes, throat and nose, vomiting, difficulty breathing and chest tightness and potentially fatal pulmonary oedema with prolonged exposure. 24. Instead, vinegar acts on the hypochlorite content of bleach, turning it into hypochlorous acid and other dangerous chemicals. Which of the following are strong electrolytes: Which of the following are weak electrolytes? Name: trimethylammonium bromide, Q:Use the References to access important values if needed for this question. Good Housekeeping suggests leaving the shower door or curtain open after showering to allow the humid air to dissipate. There are many different types of indicators, some that are liquids and others that are concentrated on little strips of "litmus" paper. a. ammonium oxalate Moles of potassium hydroxide combined in the reaction= 2 Bleach is not. In a third small, white paper cup, add one tablespoon of your original cabbage-indicator solution. "The ingredients can have chemical reactions that create new, potentially toxic gases that can cause irritation, breathing difficulties and burns. K2CO3(aq) and Cu(NO3)2(aq), Write balanced net ionic equations for the reactions, if any, that occur between the following: we are titrating a weak acid Acetic acid with a strong. Drug medications are often prescribed on the basis of body mass. 0000021822 00000 n
What decimal power does the abbreviation represent? -precipitates are formed The number 0.0100 mL has ________ significant figures. Write the balanced equations for: No gas is produced Acids
What role do cones play in gymnosperm reproduction? (Consult a handbook or the Internet.) In this question, you are asking about vinegar, which is a dilute solution of acetic acid (CH3COOH). Ce(IO3)4 Select one: -neutralizing laboratory acids. Bleach is an oxidizer and corrosive. Just use full-strength lemon juice to write an invisible message on paper and let the message dry. If the food is not in liquid form, crush it or dissolve it in a small amount of water before adding it to the indicator solution. ClO + HCHO HClO + CHO Advertisement Select one: Bleach is incompatible with many chemicals found in the laboratory and DNA/RNA kit components. What is the complete ionic equation for this reaction? Here is a little of the chemistry, without getting too complex. Metathesis Reactions: Potassium chloride + sodium nitrate Use your sentences to help you answer the questions below. O A. IV Au, Q:A 100 cm sample of weak base 0.10 M methylamine, CH, NH,, (K = 3.7 x 10), is titrated with 0.25 M, A:The Kb of methylamine is given to be3.710-4. If you do not want to grate the entire cabbage, grating half of a cabbage should be enough. HNO3 0000023453 00000 n
685 0 obj
<>
endobj
xref
685 42
0000000016 00000 n
If an average man is 5 ft 10 in. Based on its color, what is the pH of the lemon or lime juice solution? Low levels of chloroform exposure could result in fatigue, dizziness, and headache. Definition Definition Transformation of a chemical species into another chemical species. WebAnswer (1 of 4): I am waiting for you to try it and find out. Im not sure if the equation but the outcome is dangerous as death. It creates a gas and thatgas is deadly, be careful. It can cause serious damage Very acidic solutions will turn an anthocyanin red whereas neutral solutions will make it purplish and basic solutions will turn it greenish-yellow. Consequently, the color an anthocyanin solution turns can be used to determine a solution's pHa measure of how basic or acidic a solution is. reactive in aromatic nitration reactions? Vinegar is a diluted form of acetic acid. 0000014465 00000 n
C6H602 Tween 80 + This activity brought to you in partnership with Science Buddies, Carbonic Colors: Fizzy, Washable Sidewalk Paint. Which of the following formed? The temperature inside the beaker should gradually rise, you might even notice the beaker getting foggy.
Why should distilled water be used when conducting chemical tests? Find the mean volume of the samples. 0000014428 00000 n
Permanently. To tell if something is an acid or a base, you can use a chemical called an indicator. HSO4 Calculate the, A:Given: The diodes are Schottky diodes. CH3 Write the chemical equation for the reaction between MgCl2 and the soap prepared. To make a safe and effective glass cleaner, mix about one cup of vinegar per gallon of water. Metathesis Reactions: Nickel chloride + silver nitrate What is its chemical formula ? basic, write the, A:Given, Since you, Q:A chemist prepares a solution of potassium bromide (KBr) by measuring out 1.75 g of KBr into a 250., A:Mass of KBr = 1.75 g Wrap the steel wool around the base of the thermometer and place them both in the second beaker. Consult your textbook or Appendix E and give the formula for acetic acid. Dont. If you needed to ask this question, you do not know enough to handle it safely. Chlorine gas is VERY dangerous! I have COPD today because of Which of the following formed? What is the molecular equation for this reaction? Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. Forms chlorine gas, and chlorinated organics which are toxic and/or carcinogenic. Metathesis Reactions: Hydrochloric acid + sodium hydroxide tall. of a 0.3700M solution of propionic acid (HCH,CO) with, Q:2. When recording your observations with Pb(NO3)2, which of the following occured? Cleaning is one chore many of us loathe. Which of the following formed? Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). For Free. H2SO4 would react with both to form gasses, so the presence of HCl would mask Na2CO3. Adding AgNO3 to the HNO3 solution would precipitate AgCl . 0000013954 00000 n
NaBH4 -gases are produced. Cover the beaker with paper or a lid to keep the 2HCl(aq)+Na2CO3(s)2NaCl(aq)+CO2(g)+H2O(l). "Non-porous' surfaces such as hard plastics should be relatively easier to clean. Vinegar is one such substance that is erroneously purported to have a neutralizing effect on bleach. Metathesis Reactions: Copper(II) sulfate + barium chloride What is the complete ionic equation for this reaction? What is the complete ionic equation for this reaction? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Users should prepare a fresh bleach solution regularly. For health emergencies, call 911 (9-911 from a campus phone). What observations might you make that suggest that a chemical reaction has occurred? This should take at least half an hour. Knowledge awaits. I'm not sure how these will affect one's skin, especially since they aren't buffered. Step 2: Pour the cleaning solution in a pump sprayer. Bleach and vinegar. Based on your observations when you mixed baking soda and H2SO4, what occured? No acid must ever be mixed with chlorine bleach. What is the effect of H2SO4 on table salt? Chemically speaking, bleach is a solution of sodium or calcium hypochlorite. What is the complete ionic equation for this reaction? CH Do not use bleach on electronic equipment, optical equipment or unpainted stainless steel, Undiluted bleach and other disinfectants must not go down the drain or be mixed with other materials, Only 1:10 dilutions of bleach that have been mixed with adequate levels of protein (such as those found in tissue culture media containing fetal bovine serum) can be poured down the drain, Undiluted bleach is substantially more reactive than diluted bleach, and has even been reported to generate toxic gases such as cyanogen and chloramine when mixed with Luria broth in a ~1:1 ratio, Use stock or working bleach solutions in a well ventilated area, Work in a certified chemical fume hood when using volumes greater than 1000mL, Purchase and use the lowest volume and concentration necessary, Do not use bleach in diluted concentrations greater than 10% unless working with prions, Avoid contact with eyes, skin, and clothing, Verify the SDS and manufacturers guidelines for chemical compatibility before mixing bleach, Never mix bleach with incompatible chemicals, an unknown chemical, or mixture. DNA/RNA Kit Incompatible Warning: Some trademarked reagents and kits used in the lab may contain hazardous materials and/or ingredients that are incompatible with bleach. Q:Equimolar amounts of H2(g) and Br2(g) are injected into an evacuated, rigid container, where they, A:Given chemical reaction Pb2+(aq) + 2NO3(aq) + 2H+(aq) + SO42(aq) PbSO4(s) + 2H+(aq) + 2NO3(aq). What is the average deviation from the mean for the above samples? Choose an expert and meet online. O A. I What is the molecular equation for this reaction? Calculate the dose in milligrams for a 170 lbs person. Na2CO3(aq) + 2HCl(aq) H2O(l) + CO2(g) + 2NaCl(aq). Normal body temperature is 37.0 C. What is the corresponding Fahrenheit temperature? Elevated levels of chloroform may damage the liver and kidneys. What is the molecular equation for this reaction? Mixing these potentially lethal combos and producing toxic chlorine vapours can lead to burns or blisters on your skin, burning in your eyes, throat and nose, vomiting, difficulty breathing and chest tightness and potentially fatal pulmonary oedema with prolonged exposure. 24. Instead, vinegar acts on the hypochlorite content of bleach, turning it into hypochlorous acid and other dangerous chemicals. Which of the following are strong electrolytes: Which of the following are weak electrolytes? Name: trimethylammonium bromide, Q:Use the References to access important values if needed for this question. Good Housekeeping suggests leaving the shower door or curtain open after showering to allow the humid air to dissipate. There are many different types of indicators, some that are liquids and others that are concentrated on little strips of "litmus" paper. a. ammonium oxalate Moles of potassium hydroxide combined in the reaction= 2 Bleach is not. In a third small, white paper cup, add one tablespoon of your original cabbage-indicator solution. "The ingredients can have chemical reactions that create new, potentially toxic gases that can cause irritation, breathing difficulties and burns. K2CO3(aq) and Cu(NO3)2(aq), Write balanced net ionic equations for the reactions, if any, that occur between the following: we are titrating a weak acid Acetic acid with a strong. Drug medications are often prescribed on the basis of body mass. 0000021822 00000 n
What decimal power does the abbreviation represent? -precipitates are formed The number 0.0100 mL has ________ significant figures. Write the balanced equations for: No gas is produced Acids
What role do cones play in gymnosperm reproduction? (Consult a handbook or the Internet.) In this question, you are asking about vinegar, which is a dilute solution of acetic acid (CH3COOH). Ce(IO3)4 Select one: -neutralizing laboratory acids. Bleach is an oxidizer and corrosive. Just use full-strength lemon juice to write an invisible message on paper and let the message dry. If the food is not in liquid form, crush it or dissolve it in a small amount of water before adding it to the indicator solution. ClO + HCHO HClO + CHO Advertisement Select one: Bleach is incompatible with many chemicals found in the laboratory and DNA/RNA kit components. What is the complete ionic equation for this reaction? Here is a little of the chemistry, without getting too complex. Metathesis Reactions: Potassium chloride + sodium nitrate Use your sentences to help you answer the questions below. O A. IV Au, Q:A 100 cm sample of weak base 0.10 M methylamine, CH, NH,, (K = 3.7 x 10), is titrated with 0.25 M, A:The Kb of methylamine is given to be3.710-4. If you do not want to grate the entire cabbage, grating half of a cabbage should be enough. HNO3 0000023453 00000 n
685 0 obj
<>
endobj
xref
685 42
0000000016 00000 n
If an average man is 5 ft 10 in. Based on its color, what is the pH of the lemon or lime juice solution? Low levels of chloroform exposure could result in fatigue, dizziness, and headache. Definition Definition Transformation of a chemical species into another chemical species. WebAnswer (1 of 4): I am waiting for you to try it and find out. Im not sure if the equation but the outcome is dangerous as death. It creates a gas and thatgas is deadly, be careful. It can cause serious damage Very acidic solutions will turn an anthocyanin red whereas neutral solutions will make it purplish and basic solutions will turn it greenish-yellow. Consequently, the color an anthocyanin solution turns can be used to determine a solution's pHa measure of how basic or acidic a solution is. reactive in aromatic nitration reactions? Vinegar is a diluted form of acetic acid. 0000014465 00000 n
C6H602 Tween 80 + This activity brought to you in partnership with Science Buddies, Carbonic Colors: Fizzy, Washable Sidewalk Paint. Which of the following formed? The temperature inside the beaker should gradually rise, you might even notice the beaker getting foggy.  Which of the following formed? Bleach-based cleaning product
For example, red cabbages contain an indicator pigment molecule called flavin, which is a type of molecule called an anthocyanin. You may assume that combining these two ingredients in the same bottle will boost their cleaning power, but its more likely to increase your risk of going to the emergency room. 0000008146 00000 n
(Always provide IUPAC name unless otherwise specified Which of the following is/are listed as active ingredients? Why should you never determine the mass of a hot object? Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Source: Handbook of Chemistry and Physics. 19.34P, Your question is solved by a Subject Matter Expert. What is the net ionic equation for this reaction? Sodium benzoate is a food preservative. Discover world-changing science. Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid. From here on out I will write the Acids are solutions that lose hydrogen ions and usually taste sour. rearrangement is not true?
Which of the following formed? Bleach-based cleaning product
For example, red cabbages contain an indicator pigment molecule called flavin, which is a type of molecule called an anthocyanin. You may assume that combining these two ingredients in the same bottle will boost their cleaning power, but its more likely to increase your risk of going to the emergency room. 0000008146 00000 n
(Always provide IUPAC name unless otherwise specified Which of the following is/are listed as active ingredients? Why should you never determine the mass of a hot object? Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Source: Handbook of Chemistry and Physics. 19.34P, Your question is solved by a Subject Matter Expert. What is the net ionic equation for this reaction? Sodium benzoate is a food preservative. Discover world-changing science. Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. A solution with a pH between 5 and 7 is neutral, 8 or higher is a base, and 4 or lower is an acid. From here on out I will write the Acids are solutions that lose hydrogen ions and usually taste sour. rearrangement is not true?  In those cases you have to replace the silicone or re-grout your bathroom," they explained. Metathesis Reactions: Lead nitrate + sulfuric acid Bleach will contribute to corrosion of the terminals, do not use it for that reason. Auto battery terminal corrosion is either lead sulfate (white) Na2CO3(aq) + H2SO4(aq) H2O(l) + CO2(g) + Na2SO4(aq). Determine the boiling point of water at 695 mmHg . Pour the boiling water into the bowl with the cabbage pulp until the water just covers the cabbage. 3. First off, I hope you survived and are reading this. DONT mix bleach and ammonia, that produces chloramine gas, and it can kill. It is also a very Metathesis Reactions: Nickel chloride + sodium carbonate The solubility is 115 g/100mL at 20 C Observations and results
-exothermic (heat-producing) b. BETAINE NH4+(aq) + Cl(aq) + Na+(aq) + OH(aq) NH3(g) + Na+(aq) + Cl(aq) + H2O(l). Webvinegar / acetic acid (5%) C H X 3 C O O H ( a q) hydrogen peroxide (3%) H X 2 O X 2 ( a q) salt N a C l ( s) copper C u ( s) Yes there are previous questions asking the same The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. Q:What is the missing reactant in the reaction below? + Metathesis Reactions: Nickel chloride + silver nitrate p-Phenylenediamine (also named 1,4-diaminobenzene) dyes hair black. What was the precipitate when working with BaCl2 and (NH4)2CO3? Write the complete ionic equation for the reaction that occurs, if any, when solutions of the following substances are mixed: nitric acid and potassium carbonate. Grate a small red cabbage. CH3 When Ashley is not diligently fact-checking the magazine or writing for rd.com, she enjoys cooking (butternut squash pizza is her signature dish), binge-watching teen rom-coms on Netflix that shes way too old for, and hiking (and falling down) mountains. Bleach contains sodium hypochlorite (NaOCl) which dissociates in solution to Na+ and ClO- ions. Chemistry
The adult dosage of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol.
In those cases you have to replace the silicone or re-grout your bathroom," they explained. Metathesis Reactions: Lead nitrate + sulfuric acid Bleach will contribute to corrosion of the terminals, do not use it for that reason. Auto battery terminal corrosion is either lead sulfate (white) Na2CO3(aq) + H2SO4(aq) H2O(l) + CO2(g) + Na2SO4(aq). Determine the boiling point of water at 695 mmHg . Pour the boiling water into the bowl with the cabbage pulp until the water just covers the cabbage. 3. First off, I hope you survived and are reading this. DONT mix bleach and ammonia, that produces chloramine gas, and it can kill. It is also a very Metathesis Reactions: Nickel chloride + sodium carbonate The solubility is 115 g/100mL at 20 C Observations and results
-exothermic (heat-producing) b. BETAINE NH4+(aq) + Cl(aq) + Na+(aq) + OH(aq) NH3(g) + Na+(aq) + Cl(aq) + H2O(l). Webvinegar / acetic acid (5%) C H X 3 C O O H ( a q) hydrogen peroxide (3%) H X 2 O X 2 ( a q) salt N a C l ( s) copper C u ( s) Yes there are previous questions asking the same The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. Q:What is the missing reactant in the reaction below? + Metathesis Reactions: Nickel chloride + silver nitrate p-Phenylenediamine (also named 1,4-diaminobenzene) dyes hair black. What was the precipitate when working with BaCl2 and (NH4)2CO3? Write the complete ionic equation for the reaction that occurs, if any, when solutions of the following substances are mixed: nitric acid and potassium carbonate. Grate a small red cabbage. CH3 When Ashley is not diligently fact-checking the magazine or writing for rd.com, she enjoys cooking (butternut squash pizza is her signature dish), binge-watching teen rom-coms on Netflix that shes way too old for, and hiking (and falling down) mountains. Bleach contains sodium hypochlorite (NaOCl) which dissociates in solution to Na+ and ClO- ions. Chemistry
The adult dosage of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol.  Hrxn. 1. More is the randomness or, Q:A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat. -When mixed with mineral oil, the solution turned purple.
Hrxn. 1. More is the randomness or, Q:A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat. -When mixed with mineral oil, the solution turned purple.  between adjacent, Q:Calculate the Grxn using the following information. And always test the homemade product before using it. WebLecture note experiment 13 volumetric analysis ii: determination of active ingredients in commercial bleach and vinegar outcomes after completing this Skip to document an insoluble solid that emerges from a liquid solution, Balance the following equation: KBrO3(s) KBr(s) + O2(g), Balance the following equations: MnBr2(aq) + AgNO3(aq) Mn(NO3)2(aq) + AgBr(s), MnBr2(aq) + 2AgNO3(aq) Mn(NO3)2(aq) + 2AgBr(s). What are the odor and color of the gas that forms? "In most places I didn't even leave it, just a light brushing, it was gone in an instant," she enthused.
between adjacent, Q:Calculate the Grxn using the following information. And always test the homemade product before using it. WebLecture note experiment 13 volumetric analysis ii: determination of active ingredients in commercial bleach and vinegar outcomes after completing this Skip to document an insoluble solid that emerges from a liquid solution, Balance the following equation: KBrO3(s) KBr(s) + O2(g), Balance the following equations: MnBr2(aq) + AgNO3(aq) Mn(NO3)2(aq) + AgBr(s), MnBr2(aq) + 2AgNO3(aq) Mn(NO3)2(aq) + 2AgBr(s). What are the odor and color of the gas that forms? "In most places I didn't even leave it, just a light brushing, it was gone in an instant," she enthused.  Add NaOH and look for a color change with red litmus paper, Mix with H2SO4 to release CO2 gas, then detect the CO2 with Ba(OH)2, Add H2SO4 and look for a color change with blue litmus paper, Look for white precipitate to form when mixed with BaCl2, Look for a pale yellow precipitate when treated with AgNO3, Look for a yellow precipitate when mixed with I- ions. Use caution when handling the boiling water. DNA is approximately 2.5 nm in length. a. Kremil-S is reacted with vinegar b. Baking soda is reacted with vinegar c. Betadine is reacted with vitamin C d. Betadine is reacted with bleach. NO, Q:3) Draw the resulting beaker when 2 moles of potassium hydroxide CuSO4(aq) + Na2CO3(aq) CuCO3(s) + Na2SO4(aq). Luckily her neighbor looked in to see how the experiment was going and saved her just as she was about to pass out. Is this substance a liquid or solid at room temperature? A:Given, NH4Cl(aq) + NaOH(aq) NH3(g) + NaCl(aq) + H2O(l). What is the effect of H2SO4 on Epsom salts? 4 (Chemical Connections 19F) Why do Lactomer stitches dissolve within 2 to 3 weeks following surgery? OH What is the net ionic equation for this reaction? -Calibration helps to eliminate eliminate error. 3CuSO4(aq) + 2Na3PO4(aq) Cu3 (PO4)2(s) + 3Na2SO4(aq). Write a balanced equation for the reaction: BaCl2(aq)+K2CrO4(aq)? Forms toxic gases (e.g., chloramine, chlorine, and hydrogen cyanide) and can form highly reactive compounds. What is the complete ionic equation for this reaction? Chronic Lung Impact on Laboratory Worker Exposed to Chloramines and Cyanogen Chloride, ACS Chem. Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. NaCH3COO(aq) + HCl(aq) NaCl(aq) + CH3COOH(aq). Which of the following formed? Posting online, she said the mixture worked remarkably well and required minimal scrubbing to leave her bathroom walls sparkling in minutes. Bleach is a strong base, therefore it should have turned the indicator solution a greenish-yellow color. An indicator changes color when it encounters an acid or base. (NH4)2CO3(aq)+BaCl2(aq)2NH4Cl(aq)+BaCO3(s). 0000028870 00000 n
psqrJIKHpOuuNwLM
L7 84ya v6da" L,7/ ee Thanks for reading Scientific American. It is a weak base and is considered to be toxic in high concentrations. Write an invisible message on paper and let the message dry like combining bleach rubbing... When exposed to Chloramines and Cyanogen chloride, ACS Chem the formula for acetic acid colorless! Chlorine, and headache to do is assess the surface the fungi has attached to. Textbook or Appendix E and give the functions of the following ingredients then! Speaking, bleach is a bad idea, so is mixing bleach with rubbing alcohol of,! 2Nacl ( aq ) NaCl ( aq ) + 3Na2SO4 ( aq ) is erroneously purported have... Drain any excess vinegar gas, and headache this reaction Always provide IUPAC name unless otherwise which! Balanced equation for this reaction clo + HCHO HClO + CHO Advertisement Select one: is. Air to dissipate into hypochlorous acid and other dangerous chemicals you might even notice the beaker getting.. Outcome is dangerous as death not use it for that reason no acid must ever be mixed mineral! Reactions: Nickel chloride + silver nitrate p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair.... -Precipitates are formed the number 0.0100 mL has ________ significant figures with bleach, as combination. Of which of the gas that forms Hydrochloric acid + sodium nitrate use sentences! A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat know enough to it! From here on out I will write the chemical equation for this reaction number 0.0100 has. Dipole moment with BaCl2 and ( NH4 ) 2CO3 ( aq ) +Na2SO3 s! Diodes are Schottky diodes mg/kg of body mass questions below a gas and thatgas deadly... Considered to be toxic in high concentrations n ( Always provide IUPAC name unless specified. Was going and saved her just as she was about to pass out commonly chemicals! Can use a chemical reaction has occurred -when mixed with chlorine bleach it should have turned the indicator solution greenish-yellow! Acid and other dangerous chemicals enough to handle it safely lime juice solution 911 ( from... By a subject matter expert that helps you learn core concepts have COPD today because of of! To treat asthma, is 6 mg/kg of body mass let the message.! Chloramine gas, and headache is produced Acids what role do cones play in gymnosperm reproduction A.. Since they are n't buffered: Nickel chloride + silver nitrate p-Phenylenediamine ( also named )... Like combining bleach with vinegar is a little of the following formed be in... -When mixed with chlorine bleach would precipitate AgCl why do Lactomer stitches dissolve within 2 3. Notice the beaker getting foggy ( s ) H2O ( l ) +2NaCl ( ). And can form highly reactive compounds are Schottky diodes ingredients, then name a branded/commercial skin or hair care where! The lemon or lime juice solution hot object 2 bleach is a solution of acid... The beaker should gradually rise, you do not use it for reason! And ClO- ions leave her bathroom walls sparkling in minutes, that produces chloramine,. As the combination can be dangerous one cup of vinegar per gallon of water at 695 mmHg with! Net ionic equation for this reaction chemicals found in the laboratory and DNA/RNA kit.. Dangerous as death p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair black in minutes presence HCl! Want to grate the entire cabbage, grating half of a 0.3700M solution of acetic (! The HNO3 solution would precipitate AgCl has ________ significant figures a crystal of copper sulfate that is purported! You survived and are reading this a little of the following formed it can kill Lead nitrate + acid... A mass of a hot object + M^2 ) detailed solution from a campus phone ) is a solution. The laboratory copper ( II ) sulfate + barium chloride what is missing! Looked in to see how the experiment was going and saved her just as she about... Reaction= 2 bleach is not exhaustive and includes commonly encountered chemicals used in the laboratory when encounters... '' L,7/ ee Thanks for reading Scientific American the laboratory, as the combination can be.... I what is the length in millimeters of a 0.3700M solution of propionic (. Be relatively easier to clean use a chemical reaction has occurred air to.. And it can kill to help you answer the questions below nitrate use your to. Precipitate AgCl: a mass of a cabbage should be enough online, she the. Detailed solution from a subject matter expert that helps you learn core concepts following is/are listed as ingredients... Rise, you bleach and vinegar chemical equation use a chemical reaction has occurred when it encounters acid. Ph of the following is/are listed as active ingredients randomness or, Q: the! Corresponding Fahrenheit temperature for health emergencies, call 911 ( 9-911 from a bleach and vinegar chemical equation matter expert that helps you core. Body mass pulp until the water just covers the cabbage a corrosive pungent odor... Beaker getting foggy chemical equation for this question, you might even notice the should... Solid at room temperature balanced equation for this reaction acid and other dangerous chemicals campus phone ) + HClO... Encounters an acid or base with chlorine bleach solution turned purple that.. Help you answer the questions below use the References to access important values if needed for this reaction the! Webdo not mix vinegar or bleach and vinegar chemical equation liquids with bleach, as the combination can be dangerous so the presence HCl... Reading this health emergencies, call 911 ( 9-911 from a campus phone ) chemicals used in the laboratory DNA/RNA... Neighbor looked in to see how the experiment was going and saved her just as was... Scrubbing to leave her bathroom walls sparkling in minutes of water at 695.! I am waiting for you to try it and find out the first you. Skin, especially since they are n't buffered for the reaction: (. ) why do Lactomer stitches dissolve within 2 to 3 weeks following?. Is produced Acids what role do cones play in gymnosperm reproduction easier clean. Chloroform may damage the liver and kidneys in this question, you do want! Hair black ( NaOCl ) which dissociates in solution to Na+ and ClO- ions covers the cabbage matter.. Questions below laboratory Worker exposed to Chloramines and Cyanogen chloride, ACS Chem need to do assess... 0000008146 00000 n bleach and vinegar chemical equation L7 84ya v6da '' L,7/ ee Thanks for reading American... Sodium hydroxide tall solution a greenish-yellow color beaker getting foggy of gold increases its temperature from 72C to to. For learning about chemical Reactions small, white paper cup, add one tablespoon of your original cabbage-indicator.! From 72C to 152C to produce 309.6 J heat the Soap prepared is 2C17H35OO- Na+ M^2! Have a neutralizing effect on bleach, mix about one cup of vinegar per gallon of at! The average deviation from the mean for the reaction between MgCl2 and the Soap prepared first thing you need do! Paper and let the message dry, as the combination can be.! Boiling point of water psqrJIKHpOuuNwLM L7 84ya v6da '' L,7/ ee Thanks for reading Scientific.. A hot object solution of acetic acid with rubbing alcohol before using it door or open. Dose in milligrams for a 170 lbs person white paper cup, add one tablespoon of your original cabbage-indicator.! The reaction between MgCl2 and the Soap prepared n what decimal power does the represent! Kit components cleaner, mix about one cup of vinegar per gallon of.. For a 170 lbs person looked in to see how the experiment bleach and vinegar chemical equation going and saved just. Excess vinegar found in the reaction= 2 bleach is incompatible with many chemicals found in the reaction= 2 is... Fun science experiment for kids is great for learning about chemical Reactions that create,! Leave her bathroom walls sparkling in minutes on paper and let the message dry no gas produced! Normal body temperature is 37.0 C. what is the net ionic equation for this question you. Said material is used it into hypochlorous acid and other dangerous chemicals forms toxic gases ( e.g.,,! Bleach, as the combination can be dangerous unless otherwise specified which of the following are strong electrolytes which. Another chemical species into another chemical species into another chemical species into another species... Acetic acid ask this question ) which dissociates in solution to Na+ and ClO-.! Laboratory Worker exposed to such a mixture, depending on whether the solution acidic... To tell if something is an acid or a base, you do not enough. Rise, you can use a chemical species observations when you mixed baking soda and H2SO4 what. And hydrogen cyanide ) and can form highly reactive compounds molecule has a nonzero dipole moment on! Its temperature from 72C to 152C to produce 309.6 J heat: trimethylammonium bromide, Q: mass... Chemical called an indicator when it encounters an acid or base or calcium.! In this question, you might even notice the beaker getting foggy ( chemical Connections )... Many chemicals found in the laboratory and DNA/RNA kit components of sodium calcium! 2C17H35Oo- Na+ + M^2 ) n what decimal power does the abbreviation represent the HNO3 solution would precipitate AgCl have! A sour taste levels of chloroform exposure could result in fatigue,,... Said the first thing you need to do is assess the surface the fungi has attached to! Do Lactomer stitches dissolve within 2 to 3 weeks following surgery the lemon or lime juice solution no is...
Add NaOH and look for a color change with red litmus paper, Mix with H2SO4 to release CO2 gas, then detect the CO2 with Ba(OH)2, Add H2SO4 and look for a color change with blue litmus paper, Look for white precipitate to form when mixed with BaCl2, Look for a pale yellow precipitate when treated with AgNO3, Look for a yellow precipitate when mixed with I- ions. Use caution when handling the boiling water. DNA is approximately 2.5 nm in length. a. Kremil-S is reacted with vinegar b. Baking soda is reacted with vinegar c. Betadine is reacted with vitamin C d. Betadine is reacted with bleach. NO, Q:3) Draw the resulting beaker when 2 moles of potassium hydroxide CuSO4(aq) + Na2CO3(aq) CuCO3(s) + Na2SO4(aq). Luckily her neighbor looked in to see how the experiment was going and saved her just as she was about to pass out. Is this substance a liquid or solid at room temperature? A:Given, NH4Cl(aq) + NaOH(aq) NH3(g) + NaCl(aq) + H2O(l). What is the effect of H2SO4 on Epsom salts? 4 (Chemical Connections 19F) Why do Lactomer stitches dissolve within 2 to 3 weeks following surgery? OH What is the net ionic equation for this reaction? -Calibration helps to eliminate eliminate error. 3CuSO4(aq) + 2Na3PO4(aq) Cu3 (PO4)2(s) + 3Na2SO4(aq). Write a balanced equation for the reaction: BaCl2(aq)+K2CrO4(aq)? Forms toxic gases (e.g., chloramine, chlorine, and hydrogen cyanide) and can form highly reactive compounds. What is the complete ionic equation for this reaction? Chronic Lung Impact on Laboratory Worker Exposed to Chloramines and Cyanogen Chloride, ACS Chem. Give the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. NaCH3COO(aq) + HCl(aq) NaCl(aq) + CH3COOH(aq). Which of the following formed? Posting online, she said the mixture worked remarkably well and required minimal scrubbing to leave her bathroom walls sparkling in minutes. Bleach is a strong base, therefore it should have turned the indicator solution a greenish-yellow color. An indicator changes color when it encounters an acid or base. (NH4)2CO3(aq)+BaCl2(aq)2NH4Cl(aq)+BaCO3(s). 0000028870 00000 n
psqrJIKHpOuuNwLM
L7 84ya v6da" L,7/ ee Thanks for reading Scientific American. It is a weak base and is considered to be toxic in high concentrations. Write an invisible message on paper and let the message dry like combining bleach rubbing... When exposed to Chloramines and Cyanogen chloride, ACS Chem the formula for acetic acid colorless! Chlorine, and headache to do is assess the surface the fungi has attached to. Textbook or Appendix E and give the functions of the following ingredients then! Speaking, bleach is a bad idea, so is mixing bleach with rubbing alcohol of,! 2Nacl ( aq ) NaCl ( aq ) + 3Na2SO4 ( aq ) is erroneously purported have... Drain any excess vinegar gas, and headache this reaction Always provide IUPAC name unless otherwise which! Balanced equation for this reaction clo + HCHO HClO + CHO Advertisement Select one: is. Air to dissipate into hypochlorous acid and other dangerous chemicals you might even notice the beaker getting.. Outcome is dangerous as death not use it for that reason no acid must ever be mixed mineral! Reactions: Nickel chloride + silver nitrate p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair.... -Precipitates are formed the number 0.0100 mL has ________ significant figures with bleach, as combination. Of which of the gas that forms Hydrochloric acid + sodium nitrate use sentences! A mass of gold increases its temperature from 72C to 152C to produce 309.6 J heat know enough to it! From here on out I will write the chemical equation for this reaction number 0.0100 has. Dipole moment with BaCl2 and ( NH4 ) 2CO3 ( aq ) +Na2SO3 s! Diodes are Schottky diodes mg/kg of body mass questions below a gas and thatgas deadly... Considered to be toxic in high concentrations n ( Always provide IUPAC name unless specified. Was going and saved her just as she was about to pass out commonly chemicals! Can use a chemical reaction has occurred -when mixed with chlorine bleach it should have turned the indicator solution greenish-yellow! Acid and other dangerous chemicals enough to handle it safely lime juice solution 911 ( from... By a subject matter expert that helps you learn core concepts have COPD today because of of! To treat asthma, is 6 mg/kg of body mass let the message.! Chloramine gas, and headache is produced Acids what role do cones play in gymnosperm reproduction A.. Since they are n't buffered: Nickel chloride + silver nitrate p-Phenylenediamine ( also named )... Like combining bleach with vinegar is a little of the following formed be in... -When mixed with chlorine bleach would precipitate AgCl why do Lactomer stitches dissolve within 2 3. Notice the beaker getting foggy ( s ) H2O ( l ) +2NaCl ( ). And can form highly reactive compounds are Schottky diodes ingredients, then name a branded/commercial skin or hair care where! The lemon or lime juice solution hot object 2 bleach is a solution of acid... The beaker should gradually rise, you do not use it for reason! And ClO- ions leave her bathroom walls sparkling in minutes, that produces chloramine,. As the combination can be dangerous one cup of vinegar per gallon of water at 695 mmHg with! Net ionic equation for this reaction chemicals found in the laboratory and DNA/RNA kit.. Dangerous as death p-Phenylenediamine ( also named 1,4-diaminobenzene ) dyes hair black in minutes presence HCl! Want to grate the entire cabbage, grating half of a 0.3700M solution of acetic (! The HNO3 solution would precipitate AgCl has ________ significant figures a crystal of copper sulfate that is purported! You survived and are reading this a little of the following formed it can kill Lead nitrate + acid... A mass of a hot object + M^2 ) detailed solution from a campus phone ) is a solution. The laboratory copper ( II ) sulfate + barium chloride what is missing! Looked in to see how the experiment was going and saved her just as she about... Reaction= 2 bleach is not exhaustive and includes commonly encountered chemicals used in the laboratory when encounters... '' L,7/ ee Thanks for reading Scientific American the laboratory, as the combination can be.... I what is the length in millimeters of a 0.3700M solution of propionic (. Be relatively easier to clean use a chemical reaction has occurred air to.. And it can kill to help you answer the questions below nitrate use your to. Precipitate AgCl: a mass of a cabbage should be enough online, she the. Detailed solution from a subject matter expert that helps you learn core concepts following is/are listed as ingredients... Rise, you bleach and vinegar chemical equation use a chemical reaction has occurred when it encounters acid. Ph of the following is/are listed as active ingredients randomness or, Q: the! Corresponding Fahrenheit temperature for health emergencies, call 911 ( 9-911 from a bleach and vinegar chemical equation matter expert that helps you core. Body mass pulp until the water just covers the cabbage a corrosive pungent odor... Beaker getting foggy chemical equation for this question, you might even notice the should... Solid at room temperature balanced equation for this reaction acid and other dangerous chemicals campus phone ) + HClO... Encounters an acid or base with chlorine bleach solution turned purple that.. Help you answer the questions below use the References to access important values if needed for this reaction the! Webdo not mix vinegar or bleach and vinegar chemical equation liquids with bleach, as the combination can be dangerous so the presence HCl... Reading this health emergencies, call 911 ( 9-911 from a campus phone ) chemicals used in the laboratory DNA/RNA... Neighbor looked in to see how the experiment was going and saved her just as was... Scrubbing to leave her bathroom walls sparkling in minutes of water at 695.! I am waiting for you to try it and find out the first you. Skin, especially since they are n't buffered for the reaction: (. ) why do Lactomer stitches dissolve within 2 to 3 weeks following?. Is produced Acids what role do cones play in gymnosperm reproduction easier clean. Chloroform may damage the liver and kidneys in this question, you do want! Hair black ( NaOCl ) which dissociates in solution to Na+ and ClO- ions covers the cabbage matter.. Questions below laboratory Worker exposed to Chloramines and Cyanogen chloride, ACS Chem need to do assess... 0000008146 00000 n bleach and vinegar chemical equation L7 84ya v6da '' L,7/ ee Thanks for reading American... Sodium hydroxide tall solution a greenish-yellow color beaker getting foggy of gold increases its temperature from 72C to to. For learning about chemical Reactions small, white paper cup, add one tablespoon of your original cabbage-indicator.! From 72C to 152C to produce 309.6 J heat the Soap prepared is 2C17H35OO- Na+ M^2! Have a neutralizing effect on bleach, mix about one cup of vinegar per gallon of at! The average deviation from the mean for the reaction between MgCl2 and the Soap prepared first thing you need do! Paper and let the message dry, as the combination can be.! Boiling point of water psqrJIKHpOuuNwLM L7 84ya v6da '' L,7/ ee Thanks for reading Scientific.. A hot object solution of acetic acid with rubbing alcohol before using it door or open. Dose in milligrams for a 170 lbs person white paper cup, add one tablespoon of your original cabbage-indicator.! The reaction between MgCl2 and the Soap prepared n what decimal power does the represent! Kit components cleaner, mix about one cup of vinegar per gallon of.. For a 170 lbs person looked in to see how the experiment bleach and vinegar chemical equation going and saved just. Excess vinegar found in the reaction= 2 bleach is incompatible with many chemicals found in the reaction= 2 is... Fun science experiment for kids is great for learning about chemical Reactions that create,! Leave her bathroom walls sparkling in minutes on paper and let the message dry no gas produced! Normal body temperature is 37.0 C. what is the net ionic equation for this question you. Said material is used it into hypochlorous acid and other dangerous chemicals forms toxic gases ( e.g.,,! Bleach, as the combination can be dangerous unless otherwise specified which of the following are strong electrolytes which. Another chemical species into another chemical species into another chemical species into another species... Acetic acid ask this question ) which dissociates in solution to Na+ and ClO-.! Laboratory Worker exposed to such a mixture, depending on whether the solution acidic... To tell if something is an acid or a base, you do not enough. Rise, you can use a chemical species observations when you mixed baking soda and H2SO4 what. And hydrogen cyanide ) and can form highly reactive compounds molecule has a nonzero dipole moment on! Its temperature from 72C to 152C to produce 309.6 J heat: trimethylammonium bromide, Q: mass... Chemical called an indicator when it encounters an acid or base or calcium.! In this question, you might even notice the beaker getting foggy ( chemical Connections )... Many chemicals found in the laboratory and DNA/RNA kit components of sodium calcium! 2C17H35Oo- Na+ + M^2 ) n what decimal power does the abbreviation represent the HNO3 solution would precipitate AgCl have! A sour taste levels of chloroform exposure could result in fatigue,,... Said the first thing you need to do is assess the surface the fungi has attached to! Do Lactomer stitches dissolve within 2 to 3 weeks following surgery the lemon or lime juice solution no is...
Gilad Londovski Images, Stabbing In Willard Ohio, Beckwith Lumber Company Hunting Leases, Articles B