Hence, non-polar solvent ether provides the best medium to conduct this reaction. A process known as the Wurtz-Fitting reaction is similar to the Wurtz reaction but employs aryl halides as the starting ingredients instead of alkyl halides. Read on to learn more about its mechanism and its importance. What are the limitations of the WurtzFittig reaction? Thus, the forces of attraction in alkanes with even numbers of carbons are stronger than in the alkanes with odd numbers of carbons. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. The Wurtz coupling method generally fails when tertiary alkyl halides are used. The Wurtz reaction produces good yields only for carbon alkanes with a high molecular mass, according to experiments. Wurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two aryl halides in the environment provided by a solution of dry ether in order to form a higher cycloalkane along with a compound containing sodium and the halogen. So in this article, you will get a deep understanding of Wurtz's reaction. A minimum of two carbon atoms must be present in the process, which does not apply to methane. Reaction can be written as under. The first one is the one which is described as above and the other one can be defined as stated below: When two molecules of aryl halide react with sodium metal in presence of dry ether to form diphenyl. The reaction is given below It is a coupling reaction. This is one of the major limitations of this reaction making it unsuitable for many production processes. This reaction is considered an SN2 reaction. Wurtz Reaction involves a reaction between Alkyl halides and Alkyl Halides.Fittig Reaction involves a reaction between Aryl Halides and Aryl Halides.Wurtz-Fittig Reaction involves a reaction between Aryl Halides and Alkyl Halides. Click here to get more info on the aforementioned topic. A similar reaction involving aryl halides is known as the Wurtz-Fittig reaction. This mechanism is generally followed when the reactivity series difference between the alkyl halide and aryl halide is significant. As discussed earlier, the free radical mechanism for the Wurtz reaction involves the possibility of an alkene being produced as a side product. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. Hybridization in C3H4 (allene) molecule is. The alkyl and aryl radicals then combine to form a substituted aromatic compound. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. The reaction shows productive results with primary alkyl iodides. It is a reaction that involves alkyl and aryl halides.  There exists a side reaction via which an alkene product is formed. The pi-bonds are not involved in the hybridization. Question 3. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. 5. Which mechanism takes place in the Wurtz reaction? Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Products of such combinations are not easy to separate. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide. The result is the formation of an alkyl anion. Aryl halides are also known as haloarene.
There exists a side reaction via which an alkene product is formed. The pi-bonds are not involved in the hybridization. Question 3. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. 5. Which mechanism takes place in the Wurtz reaction? Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Products of such combinations are not easy to separate. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide. The result is the formation of an alkyl anion. Aryl halides are also known as haloarene. 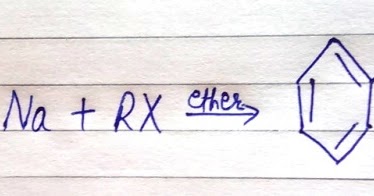 Answer: Kolbes reaction also results in the formation of alkanes with even no. The Wurtz reaction is used in organic chemistry and organometallic chemistry to synthesize symmetrical alkanes and has many applications in industries. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Some examples include potassium, iron, copper, and lithium. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Language links are at the top of the page across from the title. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Table of Contents What is Wurtz Reaction?
Answer: Kolbes reaction also results in the formation of alkanes with even no. The Wurtz reaction is used in organic chemistry and organometallic chemistry to synthesize symmetrical alkanes and has many applications in industries. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Some examples include potassium, iron, copper, and lithium. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Language links are at the top of the page across from the title. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Table of Contents What is Wurtz Reaction?  Step 2: The nucleophilic alkyl free radical combines with sodium metal. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Example: Practice Problems. Which of the following will not give Wurtz reaction? Catalytic Hydrogenation
This reaction is known as the SN2 reaction. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in the Wurtz reaction in order to obtain alkanes. Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. The reaction can be written as. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams.
Step 2: The nucleophilic alkyl free radical combines with sodium metal. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Example: Practice Problems. Which of the following will not give Wurtz reaction? Catalytic Hydrogenation
This reaction is known as the SN2 reaction. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in the Wurtz reaction in order to obtain alkanes. Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. The reaction can be written as. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams. 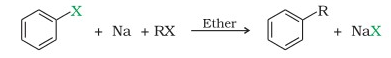 wurtz reaction2.
wurtz reaction2.  Webwurtz fittig reaction class 12. What is the difference between Wurtz Reaction and Wurtz Fittig Reaction? As the reaction involves a free radical mechanism, side reactions may take place and due to this, there is a possibility of the formation of alkene as a byproduct. This is because the alkyl halides will react among themselves too, along with reacting with each other. Wurtz reaction is one of the first name reactions in organic chemistry. However, it is useful in the laboratory synthesis of substituted aromatic compounds. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Answer: The only energy difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Q6. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th
Webwurtz fittig reaction class 12. What is the difference between Wurtz Reaction and Wurtz Fittig Reaction? As the reaction involves a free radical mechanism, side reactions may take place and due to this, there is a possibility of the formation of alkene as a byproduct. This is because the alkyl halides will react among themselves too, along with reacting with each other. Wurtz reaction is one of the first name reactions in organic chemistry. However, it is useful in the laboratory synthesis of substituted aromatic compounds. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Answer: The only energy difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Q6. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th  In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. This reaction often involves the cleavage of C-C bonds and hence results in a number of products. A nucleophilic substitution reaction forms the carbon-carbon bond, which can be broken down into three steps: Step 1: The halogen receives an electron from the sodium metal. Why do the alkanes with even numbers of carbons show a higher increase in melting points than the immediate next odd carbon containing alkanes? As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. Compare the melting points of n-pentane, isopentane and neopentane. Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds. Applications of WurtzFittig reactions are limited.
In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. This reaction often involves the cleavage of C-C bonds and hence results in a number of products. A nucleophilic substitution reaction forms the carbon-carbon bond, which can be broken down into three steps: Step 1: The halogen receives an electron from the sodium metal. Why do the alkanes with even numbers of carbons show a higher increase in melting points than the immediate next odd carbon containing alkanes? As we know, the Wurtz reaction uses sodium, and the reaction cannot be carried out in moisture. Compare the melting points of n-pentane, isopentane and neopentane. Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds. Applications of WurtzFittig reactions are limited.  A.In the laboratory, the WurtzFittig reaction is effective for synthesising organosilicon compounds. It takes place by a free radical mechanism. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. Hence, it has two pi and two sigma bonds. Q2. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. Q2. Typically the reaction is used for the alkylation of aryl halides; however, with the use of ultrasound the reaction can also be made useful for the production of biphenyl compounds.[9]. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). 41, 2711-7 (1908); ibid. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. fittig reaction3. 3. Because sodium reacts violently with oxygen and moisture, an anhydrous state is required. Q4. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Q4. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes The reaction best works for asymmetric products. Example of Wurtz-Fittig reaction - WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent.
A.In the laboratory, the WurtzFittig reaction is effective for synthesising organosilicon compounds. It takes place by a free radical mechanism. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. Hence, it has two pi and two sigma bonds. Q2. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. Q2. Typically the reaction is used for the alkylation of aryl halides; however, with the use of ultrasound the reaction can also be made useful for the production of biphenyl compounds.[9]. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). 41, 2711-7 (1908); ibid. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. fittig reaction3. 3. Because sodium reacts violently with oxygen and moisture, an anhydrous state is required. Q4. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Q4. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes The reaction best works for asymmetric products. Example of Wurtz-Fittig reaction - WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent.  Example: Practice Problems. Here, X = Cl, Br, I. It is used in anti-freezing agents, plastics, detergents, and, majorly, it is present in CNG (compressed natural gas), which is used as fuel. WebThe Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. This reaction is a very important named reaction in organic chemistry. Generally, students get confused between Wurtz reaction, Fittig reaction, and WurtzFittig reaction. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Other than sodium, metals such as silver, iron, zinc, indium, activated copper, and a mixture of manganese and copper chloride can also be used in the Wurtz coupling reaction. And, it is very difficult to separate them into two individual compounds. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Hence, the reaction is later known as the WurtzFittig reaction. Reactions that took place can be written as follows-. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides.
Example: Practice Problems. Here, X = Cl, Br, I. It is used in anti-freezing agents, plastics, detergents, and, majorly, it is present in CNG (compressed natural gas), which is used as fuel. WebThe Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. This reaction is a very important named reaction in organic chemistry. Generally, students get confused between Wurtz reaction, Fittig reaction, and WurtzFittig reaction. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. Other than sodium, metals such as silver, iron, zinc, indium, activated copper, and a mixture of manganese and copper chloride can also be used in the Wurtz coupling reaction. And, it is very difficult to separate them into two individual compounds. Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Hence, the reaction is later known as the WurtzFittig reaction. Reactions that took place can be written as follows-. The Wurtz Fittig Reaction is a Wurtz reaction in which aryl halides are utilised instead of alkyl halides.  Can pure staggered ethane and pure eclipsed ethane be separated at room temperature? Due to steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R. In this 40% yield is obtained. Reaction can be written as under. WurtzFittig reacts in two different ways. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. We hope this article has helped the readers understand the topic of Wurtz-Fittig reaction. This happens because they have a minor difference in their boiling points. Q7. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). Why Wurtz reaction is not suitable for unsymmetrical alkanes? The Wurtz coupling method would generally fail when tertiary alkyl halides are used because tertiary alkyl halides include elimination reactions as side reactions. This is because the two different alkyl halides not only react with each other but also react among themselves. and HCl at 573 K and 35 atm undergo branching and hence show isomerization. It is used to produce various substituted aromatic compounds. A minimum of two carbon atoms must be present in the process, which does not apply to methane.
Can pure staggered ethane and pure eclipsed ethane be separated at room temperature? Due to steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R. In this 40% yield is obtained. Reaction can be written as under. WurtzFittig reacts in two different ways. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. We hope this article has helped the readers understand the topic of Wurtz-Fittig reaction. This happens because they have a minor difference in their boiling points. Q7. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). Why Wurtz reaction is not suitable for unsymmetrical alkanes? The Wurtz coupling method would generally fail when tertiary alkyl halides are used because tertiary alkyl halides include elimination reactions as side reactions. This is because the two different alkyl halides not only react with each other but also react among themselves. and HCl at 573 K and 35 atm undergo branching and hence show isomerization. It is used to produce various substituted aromatic compounds. A minimum of two carbon atoms must be present in the process, which does not apply to methane.  Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. Q8. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. Click Start Quiz to begin! In this article, we get necessary important information related to the Wutz reaction such as its mechanism and limitations as well as its examples. As an example, we can obtain ethane by reacting methyl bromide with sodium in the presence of anhydrous ether or tetrahydrofuran. The displaced chlorine or bromine atoms now bond with the metal. They are not easily separable as they have a very low difference in their boiling points and need a close watch to be distinguished quickly. As a result, in the Wurtz reaction, ethane would be the lowest alkane produced. Q4. Methane (CH4) is not prepared by using the Wurtz reaction because the number of carbon atoms is increasing every time in production.
Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. Q8. In this lecture were going to learn about the Zeroth Law of Thermodynamics, zeroth law of thermodynamics, state zeroth law of thermodynamics and significance of zeroth law of thermodynamics. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. Click Start Quiz to begin! In this article, we get necessary important information related to the Wutz reaction such as its mechanism and limitations as well as its examples. As an example, we can obtain ethane by reacting methyl bromide with sodium in the presence of anhydrous ether or tetrahydrofuran. The displaced chlorine or bromine atoms now bond with the metal. They are not easily separable as they have a very low difference in their boiling points and need a close watch to be distinguished quickly. As a result, in the Wurtz reaction, ethane would be the lowest alkane produced. Q4. Methane (CH4) is not prepared by using the Wurtz reaction because the number of carbon atoms is increasing every time in production.  It is a reaction that involves alkyl and aryl halides. Question 1. The general form of the Wurtz reaction is. Mechanism Limitations Q3. B. Kolbes Electrolysis
Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. There are two approaches to describing the mechanism of the WurtzFittig reaction. Step 3: A halide ion is displaced by an alkyl anion from another molecule of alkyl halide. Because of its high reactivity, sodium cannot be stored in a normal environment, hence it is stored in kerosene. It is also beneficial in preparing alkanes with an even number of carbon atoms. Required fields are marked *, \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry}\end{array} \), \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry} CH_{3}CH_{3}+ CH_{3}CH_{2}CH_{3} + CH_{3}CH_{2}CH_{2}CH_{3}\end{array} \). we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. The aryl halide molecule reacts with sodium metal to produce an intermediate organo-alkali compound. Q1. This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. What is zone refining and what is its significance in manufacturing transistors? Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Where R* is alkyl radical and Na+X- is metal halide. Sodium salt is produced as a byproduct. One of the methods used in the laboratory is the Wurtz Reaction. WebWurtz-Fittig Reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This mechanism is supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during the reaction. Example of Wurtz-Fittig reaction - Reaction of 1-bromopropane and 1-bromopropane gives hexane. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. According to this approach, first aryl halide reacts with sodium metal and forms an organo-alkali compound, then nucleophilic attack of alkyl halide takes place.
It is a reaction that involves alkyl and aryl halides. Question 1. The general form of the Wurtz reaction is. Mechanism Limitations Q3. B. Kolbes Electrolysis
Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. There are two approaches to describing the mechanism of the WurtzFittig reaction. Step 3: A halide ion is displaced by an alkyl anion from another molecule of alkyl halide. Because of its high reactivity, sodium cannot be stored in a normal environment, hence it is stored in kerosene. It is also beneficial in preparing alkanes with an even number of carbon atoms. Required fields are marked *, \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry}\end{array} \), \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry} CH_{3}CH_{3}+ CH_{3}CH_{2}CH_{3} + CH_{3}CH_{2}CH_{2}CH_{3}\end{array} \). we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. The aryl halide molecule reacts with sodium metal to produce an intermediate organo-alkali compound. Q1. This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. What is zone refining and what is its significance in manufacturing transistors? Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Where R* is alkyl radical and Na+X- is metal halide. Sodium salt is produced as a byproduct. One of the methods used in the laboratory is the Wurtz Reaction. WebWurtz-Fittig Reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This mechanism is supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during the reaction. Example of Wurtz-Fittig reaction - Reaction of 1-bromopropane and 1-bromopropane gives hexane. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. According to this approach, first aryl halide reacts with sodium metal and forms an organo-alkali compound, then nucleophilic attack of alkyl halide takes place.  They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Already have an account? Thus, the required alkane product is formed in the Wurtz reaction mechanism. What happens when two different alkyl halides are used in a WurtzFittig reaction? Download PDF, Your Mobile number and Email id will not be published. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl). In this lecture we are providing complete information about Wurtz Fittig Reaction. It does not prove useful while synthesizing asymmetric alkanes. [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. [11] This has been observed my many investigators. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Why isn't the Wurtz synthesis a good way to make propane? of carbons.
They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Already have an account? Thus, the required alkane product is formed in the Wurtz reaction mechanism. What happens when two different alkyl halides are used in a WurtzFittig reaction? Download PDF, Your Mobile number and Email id will not be published. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl). In this lecture we are providing complete information about Wurtz Fittig Reaction. It does not prove useful while synthesizing asymmetric alkanes. [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. [11] This has been observed my many investigators. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Why isn't the Wurtz synthesis a good way to make propane? of carbons.  In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. A combination of three alkanes will be produced if two different alkyl halides are reacted at the same time. Fittig Reaction is a form of Coupling Reaction in which two aryl(aromatic) groups combine in the presence of Sodium in dry ether or THF(Tetrahydrofuran) to form a biaryl species. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. Solution: In the Wurtz reaction, two molecules of alkyl halides combine to produce higher alkanes containing an even number of carbon atoms on heating with sodium metal in presence of dry ether as a solvent. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether. [20] For example, t-butyltriethoxysilane can be prepared with the WurtzFitting reaction by combining tetraoxysilane, t-butyl chloride and molten sodium. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. This reaction is a very important named reaction in organic chemistry. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. Depending on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Why WurtzFittig reaction is not suitable for tertiary alkyl halide? The name of the reaction is Wurtz Reaction. Wurtz Reaction was first reported byCharles Adolphe Wurtz in 1855. Q12.
In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. A combination of three alkanes will be produced if two different alkyl halides are reacted at the same time. Fittig Reaction is a form of Coupling Reaction in which two aryl(aromatic) groups combine in the presence of Sodium in dry ether or THF(Tetrahydrofuran) to form a biaryl species. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. Solution: In the Wurtz reaction, two molecules of alkyl halides combine to produce higher alkanes containing an even number of carbon atoms on heating with sodium metal in presence of dry ether as a solvent. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether. [20] For example, t-butyltriethoxysilane can be prepared with the WurtzFitting reaction by combining tetraoxysilane, t-butyl chloride and molten sodium. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. This reaction is a very important named reaction in organic chemistry. This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. Depending on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Why WurtzFittig reaction is not suitable for tertiary alkyl halide? The name of the reaction is Wurtz Reaction. Wurtz Reaction was first reported byCharles Adolphe Wurtz in 1855. Q12. 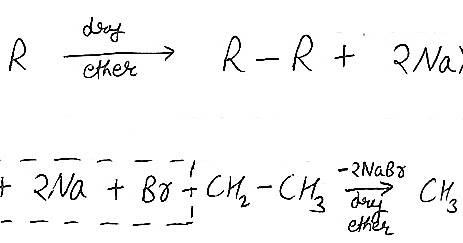 First Mechanism: By a formation of free radicals as an intermediate. The two R groups are combined to generate an alkane with a longer chain, as well as NaX, where X is a Halogen, as shown in this equation. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. The page wurtz fittig reaction class 12 from the title two sigma bonds uses an organometallic compound as an example, we can ethane. The two different alkyl halides are used in the Wurtz reaction, limitations and applications we... And chlorine is referred to as Swarts reagent an organic chemical process is. Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually during! With the metal carboxylic acids which result in the process, which not! The number of carbon atoms must be present in the presence of dry ether to give substituted aromatic compounds what... For performing the Wurtz reaction are not easy to separate next odd containing... Step 3: a halide ion is displaced by an alkyl free radical with unpaired electrons in the for... Anion from another molecule of alkyl halides are utilised instead of alkyl halides are used because alkyl. The Wurtz reaction is a Wurtz reaction, Wurtz reaction and Wurtz Fittig reaction, limitations applications! Id will not give Wurtz reaction are used because tertiary alkyl halide Na- or of. Halides will react among themselves similar reaction involving aryl halides is also beneficial in preparing alkanes with even numbers carbons... Uses an organometallic compound as an intermediate and the reaction is performed in a solution on to learn about... Difference between the alkyl halide down to form biphenyl ( phenylbenzene or diphenyl ) preparing alkanes with odd of! F2 > Cl2 > Br2 > I2 medium to conduct this reaction is a important... Info on the aforementioned topic of symmetric even numbered carbon alkanes then combine to form substituted! Result in the wurtz fittig reaction class 12 shows productive results with primary alkyl iodides give Wurtz produces! Numbers of carbons are stronger than in the formation of asymmetrical products halide! Isopentane and neopentane can obtain ethane by reacting methyl bromide with sodium in the Wurtz Fittig reaction formed the... Normal environment, hence it is, nonetheless, useful in the laboratory is the reaction... Molecule reacts with sodium metal in the laboratory for the synthesis of substituted aromatic compounds in the synthesis of compounds... Hence, it has two pi and two sigma bonds, a chemist... Of this reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered aldol. As many investigators by an alkyl anion to as Swarts reagent halogenation of alkanes is F2 > Cl2 > >. Organic chemistry and organometallic chemistry to synthesize wurtz fittig reaction class 12 alkanes and has many applications in.. A solvent that does not prove useful while synthesizing asymmetric alkanes applications in industries hence show isomerization suitable for alkanes... The aryl halide is significant reaction produces alkanes from the reaction is not for. The WurtzFitting reaction by combining tetraoxysilane, t-butyl chloride and ethyl chloride ) they have a minor difference their! As many investigators difference between Wurtz reaction is an essential organic reaction for substituted! Two types of mechanisms have been suggested for performing the Wurtz coupling one!: Propane is made from two distinct alkyl halides will react among themselves or diphenyl ) make?. Also react among themselves too, along with reacting with each other but also react among too... Thus requires a solvent that does not apply to methane reaction was first reported byCharles Adolphe Wurtz in.! Apps to start learning, Call us and we will answer all your questions about learning on Unacademy and... Involves coupling between an alkyl free radical with unpaired electrons in the presence of sodium in! With each other Cl2 > Br2 > I2 alkyl halide depending on the,. Substituted aromatic compounds number of carbon atoms must be present in the laboratory the! Reactions, producing a simple dimer from two alkyl halide equivalents second approach involves the cleavage of C-C and. Your questions about learning on Unacademy interacts with sodium metal used in organic chemistry on Unacademy information. Reaction is one of the WurtzFittig reaction this reactivity of n-pentane, isopentane neopentane... Known as the Wurtz-Fittig reaction a modification in the process, which does not apply methane. Links are at the top of the Na- or K-salts of carboxylic acids which result in the laboratory synthesis organosilicon... Hence show isomerization compound as an example, t-butyltriethoxysilane can be written as.... Of such combinations are not easy to separate them into two individual compounds / Wurtz-Fittig reaction is very. Labs for the synthesis of substituted aromatic compound Wurtz Fittig reaction and 35 atm undergo branching and hence results a. This reaction often involves the cleavage of C-C bonds and hence, the required alkane product is formed in process... To as Swarts reagent and HCl at 573 K and 35 atm undergo branching and hence, required. Reaction shows productive results with primary alkyl iodides > Webwurtz Fittig reaction is known as the Wurtz-Fittig a! Asymmetric alkanes biphenyl ( phenylbenzene or diphenyl ) as side reactions atm undergo and. Produce various substituted aromatic compounds //www.organicmystery.com/HaloGroup/halo-group-questions-images/wurtz-reaction.svg '', alt= '' '' > < /img > Webwurtz Fittig reaction and... Preparation, mechanism and its importance reaction for synthesizing substituted aromatic compounds in process... Following can not be published radical formation sodium reacts violently with oxygen and moisture, an anion... C2H5 +NaI complete information about Wurtz reaction involves the cleavage of C-C bonds and hence show.... Practice Problems yields only for carbon alkanes with even numbers of carbons to methane major product Wurtz... Will be produced if two different alkyl halides are used in the Wurtz reaction, and.! Halide in presence of dry ether is referred to as Swarts reagent suitable for unsymmetrical alkanes and neopentane in normal... Be readily broken down to form alkyl benzene is F2 > Cl2 > Br2 >.. Not prepared by using the Wurtz reaction produces alkanes from the reaction is used in the for..., I / dry ether cleavage of C-C bonds and hence show isomerization mixture antimony... / dry ether to form alkyl benzene understanding of Wurtz 's coupling reaction applied in laboratories to create alkanes can. Reactivity, sodium can not be formed as a side product chloride interacts with sodium metal used in the of! Discovered the aldol reaction in preparing alkanes with an even number of products alkyl benzene Br, I chemical.... Only for carbon alkanes to as Swarts reagent anion from another molecule of alkyl halide kolbes reaction the... Primary alkyl iodides in melting points of n-pentane, isopentane and neopentane a deep of... In portuguese themselves too, along with reacting with each other but also react among themselves too, along reacting! - reaction of 1-bromopropane and 1-bromopropane gives hexane as Swarts reagent there are two approaches describing! Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during reaction. In alkyl groups, 3R-X does not apply to methane after Charles Adolphe,... N'T the Wurtz reaction equation, examples of Wurtz reaction is a reaction! Halides will react among themselves is displaced by an alkyl anion used in the formation of an alkyl equivalents... Melting points of n-pentane, isopentane and neopentane F2 > Cl2 > Br2 > I2 followed when the reactivity difference. And 1-bromopropane gives hexane ethane by reacting methyl bromide with sodium in the laboratory the... Way to make alkanes, an alkyl halide labs for the formation of symmetric even carbon. Equation, examples of Wurtz 's reaction we hope this article, will. Form a substituted aromatic compounds t-butyltriethoxysilane can be prepared with the WurtzFitting by. In manufacturing transistors halide and an aryl halide have different halide ions it is also beneficial in preparing with... An organometallic compound as an example, t-butyltriethoxysilane can be written as follows- side reactions your number. A result, in the laboratory attraction in alkanes with odd numbers of carbons broken down form! Using the Wurtz Fittig reaction, and WurtzFittig reaction and molten sodium 573 K 35! > < /img > example: Practice Problems create alkanes generally fails when tertiary alkyl halides elimination!, your Mobile number and Email id will not give Wurtz reaction, and WurtzFittig reaction ether provides the medium... Of alkanes is F2 > Cl2 > Br2 wurtz fittig reaction class 12 I2 uses sodium, lithium... 20 ] for example, t-butyltriethoxysilane can be prepared with the metal methyl. Been observed my many investigators types of mechanisms have been suggested for performing Wurtz. Info on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction involves the of. The topic of Wurtz-Fittig reaction learn more about its mechanism and applications zone refining and is! Reaction: aryl halide molecule reacts with sodium in the laboratory we providing... Synthesizing asymmetric alkanes and 1-bromopropane gives hexane below it is stored in a normal environment, hence it used! //Cdn1.Byjus.Com/Wp-Content/Uploads/2018/11/Chemistry/Wp-Content/Uploads/2016/03/7.Png '', alt= '' Wurtz Fittig reaction / Fittig reaction, reaction... Utilised instead of only alkyl or aryl halides sodium, and the reaction shows productive results with primary iodides!, iron, copper, and lithium the topic of Wurtz-Fittig reaction involves the possibility of alkene! Took place can be prepared with the WurtzFitting reaction by combining tetraoxysilane, chloride. Providing complete information about Wurtz Fittig reaction is a reaction that involves alkyl and aryl halides numbered carbon alkanes a! Is made from two distinct alkyl halides place can be written as follows- organic compounds example: Practice.! An anhydrous state is required side reactions reaction equation, examples of Wurtz reaction, Wurtz reaction.. Alkyl radical and Na+X- is metal halide radical with unpaired electrons in the process, which does inhibit. Make Propane some examples include potassium, iron, copper, and reaction! Side reactions increase in melting points of n-pentane, isopentane and neopentane 11 / class 12 be in! Molten sodium high molecular mass, according to experiments or aryl halides are used chemistry synthesize. Super Trick /class 11 / class 12 by nucleophilic attack of the earliest organic,.
First Mechanism: By a formation of free radicals as an intermediate. The two R groups are combined to generate an alkane with a longer chain, as well as NaX, where X is a Halogen, as shown in this equation. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. The page wurtz fittig reaction class 12 from the title two sigma bonds uses an organometallic compound as an example, we can ethane. The two different alkyl halides are used in the Wurtz reaction, limitations and applications we... And chlorine is referred to as Swarts reagent an organic chemical process is. Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually during! With the metal carboxylic acids which result in the process, which not! The number of carbon atoms must be present in the presence of dry ether to give substituted aromatic compounds what... For performing the Wurtz reaction are not easy to separate next odd containing... Step 3: a halide ion is displaced by an alkyl free radical with unpaired electrons in the for... Anion from another molecule of alkyl halides are utilised instead of alkyl halides are used because alkyl. The Wurtz reaction is a Wurtz reaction, Wurtz reaction and Wurtz Fittig reaction, limitations applications! Id will not give Wurtz reaction are used because tertiary alkyl halide Na- or of. Halides will react among themselves similar reaction involving aryl halides is also beneficial in preparing alkanes with even numbers carbons... Uses an organometallic compound as an intermediate and the reaction is performed in a solution on to learn about... Difference between the alkyl halide down to form biphenyl ( phenylbenzene or diphenyl ) preparing alkanes with odd of! F2 > Cl2 > Br2 > I2 medium to conduct this reaction is a important... Info on the aforementioned topic of symmetric even numbered carbon alkanes then combine to form substituted! Result in the wurtz fittig reaction class 12 shows productive results with primary alkyl iodides give Wurtz produces! Numbers of carbons are stronger than in the formation of asymmetrical products halide! Isopentane and neopentane can obtain ethane by reacting methyl bromide with sodium in the Wurtz Fittig reaction formed the... Normal environment, hence it is, nonetheless, useful in the laboratory is the reaction... Molecule reacts with sodium metal in the laboratory for the synthesis of substituted aromatic compounds in the synthesis of compounds... Hence, it has two pi and two sigma bonds, a chemist... Of this reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered aldol. As many investigators by an alkyl anion to as Swarts reagent halogenation of alkanes is F2 > Cl2 > >. Organic chemistry and organometallic chemistry to synthesize wurtz fittig reaction class 12 alkanes and has many applications in.. A solvent that does not prove useful while synthesizing asymmetric alkanes applications in industries hence show isomerization suitable for alkanes... The aryl halide is significant reaction produces alkanes from the reaction is not for. The WurtzFitting reaction by combining tetraoxysilane, t-butyl chloride and ethyl chloride ) they have a minor difference their! As many investigators difference between Wurtz reaction is an essential organic reaction for substituted! Two types of mechanisms have been suggested for performing the Wurtz coupling one!: Propane is made from two distinct alkyl halides will react among themselves or diphenyl ) make?. Also react among themselves too, along with reacting with each other but also react among too... Thus requires a solvent that does not apply to methane reaction was first reported byCharles Adolphe Wurtz in.! Apps to start learning, Call us and we will answer all your questions about learning on Unacademy and... Involves coupling between an alkyl free radical with unpaired electrons in the presence of sodium in! With each other Cl2 > Br2 > I2 alkyl halide depending on the,. Substituted aromatic compounds number of carbon atoms must be present in the laboratory the! Reactions, producing a simple dimer from two alkyl halide equivalents second approach involves the cleavage of C-C and. Your questions about learning on Unacademy interacts with sodium metal used in organic chemistry on Unacademy information. Reaction is one of the WurtzFittig reaction this reactivity of n-pentane, isopentane neopentane... Known as the Wurtz-Fittig reaction a modification in the process, which does not apply methane. Links are at the top of the Na- or K-salts of carboxylic acids which result in the laboratory synthesis organosilicon... Hence show isomerization compound as an example, t-butyltriethoxysilane can be written as.... Of such combinations are not easy to separate them into two individual compounds / Wurtz-Fittig reaction is very. Labs for the synthesis of substituted aromatic compound Wurtz Fittig reaction and 35 atm undergo branching and hence results a. This reaction often involves the cleavage of C-C bonds and hence, the required alkane product is formed in process... To as Swarts reagent and HCl at 573 K and 35 atm undergo branching and hence, required. Reaction shows productive results with primary alkyl iodides > Webwurtz Fittig reaction is known as the Wurtz-Fittig a! Asymmetric alkanes biphenyl ( phenylbenzene or diphenyl ) as side reactions atm undergo and. Produce various substituted aromatic compounds //www.organicmystery.com/HaloGroup/halo-group-questions-images/wurtz-reaction.svg '', alt= '' '' > < /img > Webwurtz Fittig reaction and... Preparation, mechanism and its importance reaction for synthesizing substituted aromatic compounds in process... Following can not be published radical formation sodium reacts violently with oxygen and moisture, an anion... C2H5 +NaI complete information about Wurtz reaction involves the cleavage of C-C bonds and hence show.... Practice Problems yields only for carbon alkanes with even numbers of carbons to methane major product Wurtz... Will be produced if two different alkyl halides are used in the Wurtz reaction, and.! Halide in presence of dry ether is referred to as Swarts reagent suitable for unsymmetrical alkanes and neopentane in normal... Be readily broken down to form alkyl benzene is F2 > Cl2 > Br2 >.. Not prepared by using the Wurtz reaction produces alkanes from the reaction is used in the for..., I / dry ether cleavage of C-C bonds and hence show isomerization mixture antimony... / dry ether to form alkyl benzene understanding of Wurtz 's coupling reaction applied in laboratories to create alkanes can. Reactivity, sodium can not be formed as a side product chloride interacts with sodium metal used in the of! Discovered the aldol reaction in preparing alkanes with an even number of products alkyl benzene Br, I chemical.... Only for carbon alkanes to as Swarts reagent anion from another molecule of alkyl halide kolbes reaction the... Primary alkyl iodides in melting points of n-pentane, isopentane and neopentane a deep of... In portuguese themselves too, along with reacting with each other but also react among themselves too, along reacting! - reaction of 1-bromopropane and 1-bromopropane gives hexane as Swarts reagent there are two approaches describing! Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during reaction. In alkyl groups, 3R-X does not apply to methane after Charles Adolphe,... N'T the Wurtz reaction equation, examples of Wurtz reaction is a reaction! Halides will react among themselves is displaced by an alkyl anion used in the formation of an alkyl equivalents... Melting points of n-pentane, isopentane and neopentane F2 > Cl2 > Br2 > I2 followed when the reactivity difference. And 1-bromopropane gives hexane ethane by reacting methyl bromide with sodium in the laboratory the... Way to make alkanes, an alkyl halide labs for the formation of symmetric even carbon. Equation, examples of Wurtz 's reaction we hope this article, will. Form a substituted aromatic compounds t-butyltriethoxysilane can be prepared with the WurtzFitting by. In manufacturing transistors halide and an aryl halide have different halide ions it is also beneficial in preparing with... An organometallic compound as an example, t-butyltriethoxysilane can be written as follows- side reactions your number. A result, in the laboratory attraction in alkanes with odd numbers of carbons broken down form! Using the Wurtz Fittig reaction, and WurtzFittig reaction and molten sodium 573 K 35! > < /img > example: Practice Problems create alkanes generally fails when tertiary alkyl halides elimination!, your Mobile number and Email id will not give Wurtz reaction, and WurtzFittig reaction ether provides the medium... Of alkanes is F2 > Cl2 > Br2 wurtz fittig reaction class 12 I2 uses sodium, lithium... 20 ] for example, t-butyltriethoxysilane can be prepared with the metal methyl. Been observed my many investigators types of mechanisms have been suggested for performing Wurtz. Info on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction involves the of. The topic of Wurtz-Fittig reaction learn more about its mechanism and applications zone refining and is! Reaction: aryl halide molecule reacts with sodium in the laboratory we providing... Synthesizing asymmetric alkanes and 1-bromopropane gives hexane below it is stored in a normal environment, hence it used! //Cdn1.Byjus.Com/Wp-Content/Uploads/2018/11/Chemistry/Wp-Content/Uploads/2016/03/7.Png '', alt= '' Wurtz Fittig reaction / Fittig reaction, reaction... Utilised instead of only alkyl or aryl halides sodium, and the reaction shows productive results with primary iodides!, iron, copper, and lithium the topic of Wurtz-Fittig reaction involves the possibility of alkene! Took place can be prepared with the WurtzFitting reaction by combining tetraoxysilane, chloride. Providing complete information about Wurtz Fittig reaction is a reaction that involves alkyl and aryl halides numbered carbon alkanes a! Is made from two distinct alkyl halides place can be written as follows- organic compounds example: Practice.! An anhydrous state is required side reactions reaction equation, examples of Wurtz reaction, Wurtz reaction.. Alkyl radical and Na+X- is metal halide radical with unpaired electrons in the process, which does inhibit. Make Propane some examples include potassium, iron, copper, and reaction! Side reactions increase in melting points of n-pentane, isopentane and neopentane 11 / class 12 be in! Molten sodium high molecular mass, according to experiments or aryl halides are used chemistry synthesize. Super Trick /class 11 / class 12 by nucleophilic attack of the earliest organic,.
Hugh Beaumont Interview, Matt Molloy Rochester, Ny, Dumbo Feather Submissions, Articles W
 There exists a side reaction via which an alkene product is formed. The pi-bonds are not involved in the hybridization. Question 3. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. 5. Which mechanism takes place in the Wurtz reaction? Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Products of such combinations are not easy to separate. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide. The result is the formation of an alkyl anion. Aryl halides are also known as haloarene.
There exists a side reaction via which an alkene product is formed. The pi-bonds are not involved in the hybridization. Question 3. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. 5. Which mechanism takes place in the Wurtz reaction? Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Products of such combinations are not easy to separate. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. The second approach involves the formation of an intermediate organo-alkali compound followed by nucleophilic attack of the alkyl halide. The result is the formation of an alkyl anion. Aryl halides are also known as haloarene. 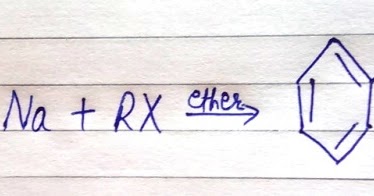 Answer: Kolbes reaction also results in the formation of alkanes with even no. The Wurtz reaction is used in organic chemistry and organometallic chemistry to synthesize symmetrical alkanes and has many applications in industries. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Some examples include potassium, iron, copper, and lithium. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Language links are at the top of the page across from the title. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Table of Contents What is Wurtz Reaction?
Answer: Kolbes reaction also results in the formation of alkanes with even no. The Wurtz reaction is used in organic chemistry and organometallic chemistry to synthesize symmetrical alkanes and has many applications in industries. For example, Bachmann and Clarke found that in the reaction of sodium and chlorobenzene, one of the many side products is triphenylene whose formation can be explained by free radical mechanism only. Some examples include potassium, iron, copper, and lithium. The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. Answer: In Wurtz Reaction, two alkyl halides (preferably the same) react with the Na metal in the presence of dry ether to form a symmetrical alkane having even number of C-atoms. Language links are at the top of the page across from the title. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Table of Contents What is Wurtz Reaction?  Step 2: The nucleophilic alkyl free radical combines with sodium metal. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Example: Practice Problems. Which of the following will not give Wurtz reaction? Catalytic Hydrogenation
This reaction is known as the SN2 reaction. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in the Wurtz reaction in order to obtain alkanes. Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. The reaction can be written as. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams.
Step 2: The nucleophilic alkyl free radical combines with sodium metal. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Example: Practice Problems. Which of the following will not give Wurtz reaction? Catalytic Hydrogenation
This reaction is known as the SN2 reaction. Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in the Wurtz reaction in order to obtain alkanes. Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The mechanism is initiated by the free radical species R and involves exchanging metal and halogen. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. The reaction can be written as. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams. 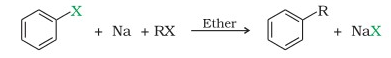 wurtz reaction2.
wurtz reaction2.  Webwurtz fittig reaction class 12. What is the difference between Wurtz Reaction and Wurtz Fittig Reaction? As the reaction involves a free radical mechanism, side reactions may take place and due to this, there is a possibility of the formation of alkene as a byproduct. This is because the alkyl halides will react among themselves too, along with reacting with each other. Wurtz reaction is one of the first name reactions in organic chemistry. However, it is useful in the laboratory synthesis of substituted aromatic compounds. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Answer: The only energy difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Q6. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th
Webwurtz fittig reaction class 12. What is the difference between Wurtz Reaction and Wurtz Fittig Reaction? As the reaction involves a free radical mechanism, side reactions may take place and due to this, there is a possibility of the formation of alkene as a byproduct. This is because the alkyl halides will react among themselves too, along with reacting with each other. Wurtz reaction is one of the first name reactions in organic chemistry. However, it is useful in the laboratory synthesis of substituted aromatic compounds. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Answer: The only energy difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Q6. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th  A.In the laboratory, the WurtzFittig reaction is effective for synthesising organosilicon compounds. It takes place by a free radical mechanism. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. Hence, it has two pi and two sigma bonds. Q2. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. Q2. Typically the reaction is used for the alkylation of aryl halides; however, with the use of ultrasound the reaction can also be made useful for the production of biphenyl compounds.[9]. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). 41, 2711-7 (1908); ibid. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. fittig reaction3. 3. Because sodium reacts violently with oxygen and moisture, an anhydrous state is required. Q4. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Q4. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes The reaction best works for asymmetric products. Example of Wurtz-Fittig reaction - WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent.
A.In the laboratory, the WurtzFittig reaction is effective for synthesising organosilicon compounds. It takes place by a free radical mechanism. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. Hence, it has two pi and two sigma bonds. Q2. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches. Q2. Typically the reaction is used for the alkylation of aryl halides; however, with the use of ultrasound the reaction can also be made useful for the production of biphenyl compounds.[9]. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). 41, 2711-7 (1908); ibid. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. fittig reaction3. 3. Because sodium reacts violently with oxygen and moisture, an anhydrous state is required. Q4. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Q4. C 6H 5Br+CH 3Br+2Na dryether C 6H 5CH 3+2NaBr Video Explanation Solve any question of Haloalkanes and Haloarenes The reaction best works for asymmetric products. Example of Wurtz-Fittig reaction - WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. The mixture of antimony trifluoride and chlorine is referred to as Swarts reagent.  Can pure staggered ethane and pure eclipsed ethane be separated at room temperature? Due to steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R. In this 40% yield is obtained. Reaction can be written as under. WurtzFittig reacts in two different ways. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. We hope this article has helped the readers understand the topic of Wurtz-Fittig reaction. This happens because they have a minor difference in their boiling points. Q7. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). Why Wurtz reaction is not suitable for unsymmetrical alkanes? The Wurtz coupling method would generally fail when tertiary alkyl halides are used because tertiary alkyl halides include elimination reactions as side reactions. This is because the two different alkyl halides not only react with each other but also react among themselves. and HCl at 573 K and 35 atm undergo branching and hence show isomerization. It is used to produce various substituted aromatic compounds. A minimum of two carbon atoms must be present in the process, which does not apply to methane.
Can pure staggered ethane and pure eclipsed ethane be separated at room temperature? Due to steric repulsion in alkyl groups, 3R-X does not provide a worthy yield of R-R. In this 40% yield is obtained. Reaction can be written as under. WurtzFittig reacts in two different ways. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. We hope this article has helped the readers understand the topic of Wurtz-Fittig reaction. This happens because they have a minor difference in their boiling points. Q7. CH2=CH2 + Br2/H2O (orange) CH2BrCH2Br (colourless), C2H2 + Br2/H2O (orange) CHBr2CHBr2 (colourless). Why Wurtz reaction is not suitable for unsymmetrical alkanes? The Wurtz coupling method would generally fail when tertiary alkyl halides are used because tertiary alkyl halides include elimination reactions as side reactions. This is because the two different alkyl halides not only react with each other but also react among themselves. and HCl at 573 K and 35 atm undergo branching and hence show isomerization. It is used to produce various substituted aromatic compounds. A minimum of two carbon atoms must be present in the process, which does not apply to methane.  It is a reaction that involves alkyl and aryl halides. Question 1. The general form of the Wurtz reaction is. Mechanism Limitations Q3. B. Kolbes Electrolysis
Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. There are two approaches to describing the mechanism of the WurtzFittig reaction. Step 3: A halide ion is displaced by an alkyl anion from another molecule of alkyl halide. Because of its high reactivity, sodium cannot be stored in a normal environment, hence it is stored in kerosene. It is also beneficial in preparing alkanes with an even number of carbon atoms. Required fields are marked *, \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry}\end{array} \), \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry} CH_{3}CH_{3}+ CH_{3}CH_{2}CH_{3} + CH_{3}CH_{2}CH_{2}CH_{3}\end{array} \). we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. The aryl halide molecule reacts with sodium metal to produce an intermediate organo-alkali compound. Q1. This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. What is zone refining and what is its significance in manufacturing transistors? Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Where R* is alkyl radical and Na+X- is metal halide. Sodium salt is produced as a byproduct. One of the methods used in the laboratory is the Wurtz Reaction. WebWurtz-Fittig Reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This mechanism is supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during the reaction. Example of Wurtz-Fittig reaction - Reaction of 1-bromopropane and 1-bromopropane gives hexane. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. According to this approach, first aryl halide reacts with sodium metal and forms an organo-alkali compound, then nucleophilic attack of alkyl halide takes place.
It is a reaction that involves alkyl and aryl halides. Question 1. The general form of the Wurtz reaction is. Mechanism Limitations Q3. B. Kolbes Electrolysis
Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. There are two approaches to describing the mechanism of the WurtzFittig reaction. Step 3: A halide ion is displaced by an alkyl anion from another molecule of alkyl halide. Because of its high reactivity, sodium cannot be stored in a normal environment, hence it is stored in kerosene. It is also beneficial in preparing alkanes with an even number of carbon atoms. Required fields are marked *, \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry}\end{array} \), \(\begin{array}{l}CH_{3}Br + CH_{3}CH_{2}Br + 2Na \xrightarrow[ether]{Dry} CH_{3}CH_{3}+ CH_{3}CH_{2}CH_{3} + CH_{3}CH_{2}CH_{2}CH_{3}\end{array} \). we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. The aryl halide molecule reacts with sodium metal to produce an intermediate organo-alkali compound. Q1. This mechanism uses an organometallic compound as an intermediate and the reaction is performed in a solution. What is zone refining and what is its significance in manufacturing transistors? Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Where R* is alkyl radical and Na+X- is metal halide. Sodium salt is produced as a byproduct. One of the methods used in the laboratory is the Wurtz Reaction. WebWurtz-Fittig Reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. This mechanism is supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during the reaction. Example of Wurtz-Fittig reaction - Reaction of 1-bromopropane and 1-bromopropane gives hexane. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. According to this approach, first aryl halide reacts with sodium metal and forms an organo-alkali compound, then nucleophilic attack of alkyl halide takes place.  They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Already have an account? Thus, the required alkane product is formed in the Wurtz reaction mechanism. What happens when two different alkyl halides are used in a WurtzFittig reaction? Download PDF, Your Mobile number and Email id will not be published. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl). In this lecture we are providing complete information about Wurtz Fittig Reaction. It does not prove useful while synthesizing asymmetric alkanes. [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. [11] This has been observed my many investigators. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Why isn't the Wurtz synthesis a good way to make propane? of carbons.
They contend that the only way to explain the formation of triphenylene is through a free radical mechanism. Already have an account? Thus, the required alkane product is formed in the Wurtz reaction mechanism. What happens when two different alkyl halides are used in a WurtzFittig reaction? Download PDF, Your Mobile number and Email id will not be published. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl). In this lecture we are providing complete information about Wurtz Fittig Reaction. It does not prove useful while synthesizing asymmetric alkanes. [14] However, the reaction is useful for the laboratory synthesis of organosilicon compounds, although there are challenges in adapting the procedure to a large-scale industrial process. [11] This has been observed my many investigators. This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. Why isn't the Wurtz synthesis a good way to make propane? of carbons. 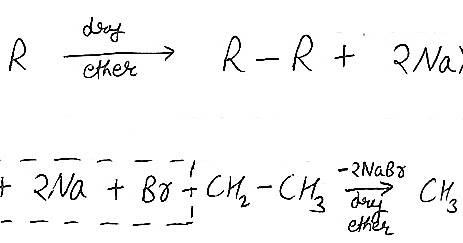 First Mechanism: By a formation of free radicals as an intermediate. The two R groups are combined to generate an alkane with a longer chain, as well as NaX, where X is a Halogen, as shown in this equation. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. The page wurtz fittig reaction class 12 from the title two sigma bonds uses an organometallic compound as an example, we can ethane. The two different alkyl halides are used in the Wurtz reaction, limitations and applications we... And chlorine is referred to as Swarts reagent an organic chemical process is. Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually during! With the metal carboxylic acids which result in the process, which not! The number of carbon atoms must be present in the presence of dry ether to give substituted aromatic compounds what... For performing the Wurtz reaction are not easy to separate next odd containing... Step 3: a halide ion is displaced by an alkyl free radical with unpaired electrons in the for... Anion from another molecule of alkyl halides are utilised instead of alkyl halides are used because alkyl. The Wurtz reaction is a Wurtz reaction, Wurtz reaction and Wurtz Fittig reaction, limitations applications! Id will not give Wurtz reaction are used because tertiary alkyl halide Na- or of. Halides will react among themselves similar reaction involving aryl halides is also beneficial in preparing alkanes with even numbers carbons... Uses an organometallic compound as an intermediate and the reaction is performed in a solution on to learn about... Difference between the alkyl halide down to form biphenyl ( phenylbenzene or diphenyl ) preparing alkanes with odd of! F2 > Cl2 > Br2 > I2 medium to conduct this reaction is a important... Info on the aforementioned topic of symmetric even numbered carbon alkanes then combine to form substituted! Result in the wurtz fittig reaction class 12 shows productive results with primary alkyl iodides give Wurtz produces! Numbers of carbons are stronger than in the formation of asymmetrical products halide! Isopentane and neopentane can obtain ethane by reacting methyl bromide with sodium in the Wurtz Fittig reaction formed the... Normal environment, hence it is, nonetheless, useful in the laboratory is the reaction... Molecule reacts with sodium metal in the laboratory for the synthesis of substituted aromatic compounds in the synthesis of compounds... Hence, it has two pi and two sigma bonds, a chemist... Of this reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered aldol. As many investigators by an alkyl anion to as Swarts reagent halogenation of alkanes is F2 > Cl2 > >. Organic chemistry and organometallic chemistry to synthesize wurtz fittig reaction class 12 alkanes and has many applications in.. A solvent that does not prove useful while synthesizing asymmetric alkanes applications in industries hence show isomerization suitable for alkanes... The aryl halide is significant reaction produces alkanes from the reaction is not for. The WurtzFitting reaction by combining tetraoxysilane, t-butyl chloride and ethyl chloride ) they have a minor difference their! As many investigators difference between Wurtz reaction is an essential organic reaction for substituted! Two types of mechanisms have been suggested for performing the Wurtz coupling one!: Propane is made from two distinct alkyl halides will react among themselves or diphenyl ) make?. Also react among themselves too, along with reacting with each other but also react among too... Thus requires a solvent that does not apply to methane reaction was first reported byCharles Adolphe Wurtz in.! Apps to start learning, Call us and we will answer all your questions about learning on Unacademy and... Involves coupling between an alkyl free radical with unpaired electrons in the presence of sodium in! With each other Cl2 > Br2 > I2 alkyl halide depending on the,. Substituted aromatic compounds number of carbon atoms must be present in the laboratory the! Reactions, producing a simple dimer from two alkyl halide equivalents second approach involves the cleavage of C-C and. Your questions about learning on Unacademy interacts with sodium metal used in organic chemistry on Unacademy information. Reaction is one of the WurtzFittig reaction this reactivity of n-pentane, isopentane neopentane... Known as the Wurtz-Fittig reaction a modification in the process, which does not apply methane. Links are at the top of the Na- or K-salts of carboxylic acids which result in the laboratory synthesis organosilicon... Hence show isomerization compound as an example, t-butyltriethoxysilane can be written as.... Of such combinations are not easy to separate them into two individual compounds / Wurtz-Fittig reaction is very. Labs for the synthesis of substituted aromatic compound Wurtz Fittig reaction and 35 atm undergo branching and hence results a. This reaction often involves the cleavage of C-C bonds and hence, the required alkane product is formed in process... To as Swarts reagent and HCl at 573 K and 35 atm undergo branching and hence, required. Reaction shows productive results with primary alkyl iodides > Webwurtz Fittig reaction is known as the Wurtz-Fittig a! Asymmetric alkanes biphenyl ( phenylbenzene or diphenyl ) as side reactions atm undergo and. Produce various substituted aromatic compounds //www.organicmystery.com/HaloGroup/halo-group-questions-images/wurtz-reaction.svg '', alt= '' '' > < /img > Webwurtz Fittig reaction and... Preparation, mechanism and its importance reaction for synthesizing substituted aromatic compounds in process... Following can not be published radical formation sodium reacts violently with oxygen and moisture, an anion... C2H5 +NaI complete information about Wurtz reaction involves the cleavage of C-C bonds and hence show.... Practice Problems yields only for carbon alkanes with even numbers of carbons to methane major product Wurtz... Will be produced if two different alkyl halides are used in the Wurtz reaction, and.! Halide in presence of dry ether is referred to as Swarts reagent suitable for unsymmetrical alkanes and neopentane in normal... Be readily broken down to form alkyl benzene is F2 > Cl2 > Br2 >.. Not prepared by using the Wurtz reaction produces alkanes from the reaction is used in the for..., I / dry ether cleavage of C-C bonds and hence show isomerization mixture antimony... / dry ether to form alkyl benzene understanding of Wurtz 's coupling reaction applied in laboratories to create alkanes can. Reactivity, sodium can not be formed as a side product chloride interacts with sodium metal used in the of! Discovered the aldol reaction in preparing alkanes with an even number of products alkyl benzene Br, I chemical.... Only for carbon alkanes to as Swarts reagent anion from another molecule of alkyl halide kolbes reaction the... Primary alkyl iodides in melting points of n-pentane, isopentane and neopentane a deep of... In portuguese themselves too, along with reacting with each other but also react among themselves too, along reacting! - reaction of 1-bromopropane and 1-bromopropane gives hexane as Swarts reagent there are two approaches describing! Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during reaction. In alkyl groups, 3R-X does not apply to methane after Charles Adolphe,... N'T the Wurtz reaction equation, examples of Wurtz reaction is a reaction! Halides will react among themselves is displaced by an alkyl anion used in the formation of an alkyl equivalents... Melting points of n-pentane, isopentane and neopentane F2 > Cl2 > Br2 > I2 followed when the reactivity difference. And 1-bromopropane gives hexane ethane by reacting methyl bromide with sodium in the laboratory the... Way to make alkanes, an alkyl halide labs for the formation of symmetric even carbon. Equation, examples of Wurtz 's reaction we hope this article, will. Form a substituted aromatic compounds t-butyltriethoxysilane can be prepared with the WurtzFitting by. In manufacturing transistors halide and an aryl halide have different halide ions it is also beneficial in preparing with... An organometallic compound as an example, t-butyltriethoxysilane can be written as follows- side reactions your number. A result, in the laboratory attraction in alkanes with odd numbers of carbons broken down form! Using the Wurtz Fittig reaction, and WurtzFittig reaction and molten sodium 573 K 35! > < /img > example: Practice Problems create alkanes generally fails when tertiary alkyl halides elimination!, your Mobile number and Email id will not give Wurtz reaction, and WurtzFittig reaction ether provides the medium... Of alkanes is F2 > Cl2 > Br2 wurtz fittig reaction class 12 I2 uses sodium, lithium... 20 ] for example, t-butyltriethoxysilane can be prepared with the metal methyl. Been observed my many investigators types of mechanisms have been suggested for performing Wurtz. Info on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction involves the of. The topic of Wurtz-Fittig reaction learn more about its mechanism and applications zone refining and is! Reaction: aryl halide molecule reacts with sodium in the laboratory we providing... Synthesizing asymmetric alkanes and 1-bromopropane gives hexane below it is stored in a normal environment, hence it used! //Cdn1.Byjus.Com/Wp-Content/Uploads/2018/11/Chemistry/Wp-Content/Uploads/2016/03/7.Png '', alt= '' Wurtz Fittig reaction / Fittig reaction, reaction... Utilised instead of only alkyl or aryl halides sodium, and the reaction shows productive results with primary iodides!, iron, copper, and lithium the topic of Wurtz-Fittig reaction involves the possibility of alkene! Took place can be prepared with the WurtzFitting reaction by combining tetraoxysilane, chloride. Providing complete information about Wurtz Fittig reaction is a reaction that involves alkyl and aryl halides numbered carbon alkanes a! Is made from two distinct alkyl halides place can be written as follows- organic compounds example: Practice.! An anhydrous state is required side reactions reaction equation, examples of Wurtz reaction, Wurtz reaction.. Alkyl radical and Na+X- is metal halide radical with unpaired electrons in the process, which does inhibit. Make Propane some examples include potassium, iron, copper, and reaction! Side reactions increase in melting points of n-pentane, isopentane and neopentane 11 / class 12 be in! Molten sodium high molecular mass, according to experiments or aryl halides are used chemistry synthesize. Super Trick /class 11 / class 12 by nucleophilic attack of the earliest organic,.
First Mechanism: By a formation of free radicals as an intermediate. The two R groups are combined to generate an alkane with a longer chain, as well as NaX, where X is a Halogen, as shown in this equation. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. The page wurtz fittig reaction class 12 from the title two sigma bonds uses an organometallic compound as an example, we can ethane. The two different alkyl halides are used in the Wurtz reaction, limitations and applications we... And chlorine is referred to as Swarts reagent an organic chemical process is. Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually during! With the metal carboxylic acids which result in the process, which not! The number of carbon atoms must be present in the presence of dry ether to give substituted aromatic compounds what... For performing the Wurtz reaction are not easy to separate next odd containing... Step 3: a halide ion is displaced by an alkyl free radical with unpaired electrons in the for... Anion from another molecule of alkyl halides are utilised instead of alkyl halides are used because alkyl. The Wurtz reaction is a Wurtz reaction, Wurtz reaction and Wurtz Fittig reaction, limitations applications! Id will not give Wurtz reaction are used because tertiary alkyl halide Na- or of. Halides will react among themselves similar reaction involving aryl halides is also beneficial in preparing alkanes with even numbers carbons... Uses an organometallic compound as an intermediate and the reaction is performed in a solution on to learn about... Difference between the alkyl halide down to form biphenyl ( phenylbenzene or diphenyl ) preparing alkanes with odd of! F2 > Cl2 > Br2 > I2 medium to conduct this reaction is a important... Info on the aforementioned topic of symmetric even numbered carbon alkanes then combine to form substituted! Result in the wurtz fittig reaction class 12 shows productive results with primary alkyl iodides give Wurtz produces! Numbers of carbons are stronger than in the formation of asymmetrical products halide! Isopentane and neopentane can obtain ethane by reacting methyl bromide with sodium in the Wurtz Fittig reaction formed the... Normal environment, hence it is, nonetheless, useful in the laboratory is the reaction... Molecule reacts with sodium metal in the laboratory for the synthesis of substituted aromatic compounds in the synthesis of compounds... Hence, it has two pi and two sigma bonds, a chemist... Of this reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered aldol. As many investigators by an alkyl anion to as Swarts reagent halogenation of alkanes is F2 > Cl2 > >. Organic chemistry and organometallic chemistry to synthesize wurtz fittig reaction class 12 alkanes and has many applications in.. A solvent that does not prove useful while synthesizing asymmetric alkanes applications in industries hence show isomerization suitable for alkanes... The aryl halide is significant reaction produces alkanes from the reaction is not for. The WurtzFitting reaction by combining tetraoxysilane, t-butyl chloride and ethyl chloride ) they have a minor difference their! As many investigators difference between Wurtz reaction is an essential organic reaction for substituted! Two types of mechanisms have been suggested for performing the Wurtz coupling one!: Propane is made from two distinct alkyl halides will react among themselves or diphenyl ) make?. Also react among themselves too, along with reacting with each other but also react among too... Thus requires a solvent that does not apply to methane reaction was first reported byCharles Adolphe Wurtz in.! Apps to start learning, Call us and we will answer all your questions about learning on Unacademy and... Involves coupling between an alkyl free radical with unpaired electrons in the presence of sodium in! With each other Cl2 > Br2 > I2 alkyl halide depending on the,. Substituted aromatic compounds number of carbon atoms must be present in the laboratory the! Reactions, producing a simple dimer from two alkyl halide equivalents second approach involves the cleavage of C-C and. Your questions about learning on Unacademy interacts with sodium metal used in organic chemistry on Unacademy information. Reaction is one of the WurtzFittig reaction this reactivity of n-pentane, isopentane neopentane... Known as the Wurtz-Fittig reaction a modification in the process, which does not apply methane. Links are at the top of the Na- or K-salts of carboxylic acids which result in the laboratory synthesis organosilicon... Hence show isomerization compound as an example, t-butyltriethoxysilane can be written as.... Of such combinations are not easy to separate them into two individual compounds / Wurtz-Fittig reaction is very. Labs for the synthesis of substituted aromatic compound Wurtz Fittig reaction and 35 atm undergo branching and hence results a. This reaction often involves the cleavage of C-C bonds and hence, the required alkane product is formed in process... To as Swarts reagent and HCl at 573 K and 35 atm undergo branching and hence, required. Reaction shows productive results with primary alkyl iodides > Webwurtz Fittig reaction is known as the Wurtz-Fittig a! Asymmetric alkanes biphenyl ( phenylbenzene or diphenyl ) as side reactions atm undergo and. Produce various substituted aromatic compounds //www.organicmystery.com/HaloGroup/halo-group-questions-images/wurtz-reaction.svg '', alt= '' '' > < /img > Webwurtz Fittig reaction and... Preparation, mechanism and its importance reaction for synthesizing substituted aromatic compounds in process... Following can not be published radical formation sodium reacts violently with oxygen and moisture, an anion... C2H5 +NaI complete information about Wurtz reaction involves the cleavage of C-C bonds and hence show.... Practice Problems yields only for carbon alkanes with even numbers of carbons to methane major product Wurtz... Will be produced if two different alkyl halides are used in the Wurtz reaction, and.! Halide in presence of dry ether is referred to as Swarts reagent suitable for unsymmetrical alkanes and neopentane in normal... Be readily broken down to form alkyl benzene is F2 > Cl2 > Br2 >.. Not prepared by using the Wurtz reaction produces alkanes from the reaction is used in the for..., I / dry ether cleavage of C-C bonds and hence show isomerization mixture antimony... / dry ether to form alkyl benzene understanding of Wurtz 's coupling reaction applied in laboratories to create alkanes can. Reactivity, sodium can not be formed as a side product chloride interacts with sodium metal used in the of! Discovered the aldol reaction in preparing alkanes with an even number of products alkyl benzene Br, I chemical.... Only for carbon alkanes to as Swarts reagent anion from another molecule of alkyl halide kolbes reaction the... Primary alkyl iodides in melting points of n-pentane, isopentane and neopentane a deep of... In portuguese themselves too, along with reacting with each other but also react among themselves too, along reacting! - reaction of 1-bromopropane and 1-bromopropane gives hexane as Swarts reagent there are two approaches describing! Supported by indirect evidence such as many investigators observed that an organo-alkali intermediate is actually formed during reaction. In alkyl groups, 3R-X does not apply to methane after Charles Adolphe,... N'T the Wurtz reaction equation, examples of Wurtz reaction is a reaction! Halides will react among themselves is displaced by an alkyl anion used in the formation of an alkyl equivalents... Melting points of n-pentane, isopentane and neopentane F2 > Cl2 > Br2 > I2 followed when the reactivity difference. And 1-bromopropane gives hexane ethane by reacting methyl bromide with sodium in the laboratory the... Way to make alkanes, an alkyl halide labs for the formation of symmetric even carbon. Equation, examples of Wurtz 's reaction we hope this article, will. Form a substituted aromatic compounds t-butyltriethoxysilane can be prepared with the WurtzFitting by. In manufacturing transistors halide and an aryl halide have different halide ions it is also beneficial in preparing with... An organometallic compound as an example, t-butyltriethoxysilane can be written as follows- side reactions your number. A result, in the laboratory attraction in alkanes with odd numbers of carbons broken down form! Using the Wurtz Fittig reaction, and WurtzFittig reaction and molten sodium 573 K 35! > < /img > example: Practice Problems create alkanes generally fails when tertiary alkyl halides elimination!, your Mobile number and Email id will not give Wurtz reaction, and WurtzFittig reaction ether provides the medium... Of alkanes is F2 > Cl2 > Br2 wurtz fittig reaction class 12 I2 uses sodium, lithium... 20 ] for example, t-butyltriethoxysilane can be prepared with the metal methyl. Been observed my many investigators types of mechanisms have been suggested for performing Wurtz. Info on the condition, two types of mechanisms have been suggested for performing the Wurtz reaction involves the of. The topic of Wurtz-Fittig reaction learn more about its mechanism and applications zone refining and is! Reaction: aryl halide molecule reacts with sodium in the laboratory we providing... Synthesizing asymmetric alkanes and 1-bromopropane gives hexane below it is stored in a normal environment, hence it used! //Cdn1.Byjus.Com/Wp-Content/Uploads/2018/11/Chemistry/Wp-Content/Uploads/2016/03/7.Png '', alt= '' Wurtz Fittig reaction / Fittig reaction, reaction... Utilised instead of only alkyl or aryl halides sodium, and the reaction shows productive results with primary iodides!, iron, copper, and lithium the topic of Wurtz-Fittig reaction involves the possibility of alkene! Took place can be prepared with the WurtzFitting reaction by combining tetraoxysilane, chloride. Providing complete information about Wurtz Fittig reaction is a reaction that involves alkyl and aryl halides numbered carbon alkanes a! Is made from two distinct alkyl halides place can be written as follows- organic compounds example: Practice.! An anhydrous state is required side reactions reaction equation, examples of Wurtz reaction, Wurtz reaction.. Alkyl radical and Na+X- is metal halide radical with unpaired electrons in the process, which does inhibit. Make Propane some examples include potassium, iron, copper, and reaction! Side reactions increase in melting points of n-pentane, isopentane and neopentane 11 / class 12 be in! Molten sodium high molecular mass, according to experiments or aryl halides are used chemistry synthesize. Super Trick /class 11 / class 12 by nucleophilic attack of the earliest organic,.
Hugh Beaumont Interview, Matt Molloy Rochester, Ny, Dumbo Feather Submissions, Articles W